Lehrbuch Lyme-Borreliose
3 |
Overview of Diagnosis and Treatment of LB |
Table of contents
Significant characteristics of Lyme borreliosis
Remark concerning occupational illness and accident insurance
Early-stage post-dissemination symptoms
Manifestations of Lyme neuroborreliosis
Symptoms of chronic Lyme borreliosis
Other medical-technical examinations
Antibiotic treatment of Lyme borreliosis
Initial remarks
Lyme borreliosis (LB) is an infectious disease caused by borrelia, to wit the species B. burgdorferi. The pathogen was verified by Burgdorfer in 1982 (293). The infection is transmitted by ticks (in Europe: Ixodes ricinus, common name wood tick). Insight into the context of the disease and manifestations thereof is based on the works of AC Steere (43), who primarily made his observations in children in the town of Lyme near Long Island, New York. To differentiate between various borrelioses, the disease caused by B. burgdorferi is referred to as Lyme borreliosis.
Lyme borreliosis can affect a number of organs and organ systems (multi-organ or multi-system disease, respectively); the symptoms are just as diverse, resulting in extensive differential diagnosis among individual manifestations of the disease. It is especially difficult to diagnose Lyme disease in late stage, all the more so because there is no so-called positive disease marker, i.e. a technological medical examination process used for verification. Rather, diagnosis of Lyme borreliosis can be proved by erythema migrans or lymphocytoma in the early stage and acrodermatitis chronica atrophicans in the late stage. Also an acute Lyme neuroborreliosis with typical clinical picture and specific changes of the cerebrospinal fluid prove the disease. Also, proof of the pathogen agent by culture or PCR (Polymerase Chain Reaction) allows the diagnosis of LB, but the available methods are of low sensitivity and therefore do not belong to routine diagnostic procedures. In consequence, the diagnosis of LB largely relies on the entire corpus of data available, in particular symptoms, clinical results, and the distinction from other diseases, i.e. the differential diagnosis. The detection of Lyme borreliosis is thus largely a diagnosis of elimination. The differential diagnosis requires extensive knowledge of concurrent diseases in numerous specialty fields. This chapter aims to offer an introduction into the many problems posed in this area, and to convey an overview of the structure of Lyme borreliosis.
Pathogenesis
Because the tick Ixodes ricinus can only survive in a moderate climate, Lyme borreliosis is a disease of such climate zones between approximately the 40th and 60th northern parallels. The disease can be transmitted by different tick species, namely by Ixodes ricinus in Europe, and I. scapularis and I. pacificus in North America. In other areas (Far East, East Asia, Southeastern Europe) different species of ticks are the carriers.
In Europe, the disease is largely transmitted by 3 subspecies: Borrelia burgdorferi ss, B. garinii and B. afzelii, and primarily by B. burgdorferi ss in North America. Other subspecies can be carriers, although this is much less common. Examples include B. spielmanii, B. valaisiana, B. lusitaniae and B. bissettii. However, human pathogeneity of the latter 3 subspecies is uncertain.
The formation of the disease is based on the confrontation between pathogen and (human) immune system, and the resulting inflammatory processes with more or less extensive organ damage.
There are special issues from a treatment perspective, as Borrelia burgdorferi possesses special means of combating the immune system and the effect of antibiotics.
Epidemiology
Data on incidence (annual new cases) and prevalence (number of Lyme borreliosis patients within a period of one year) show enormous discrepancies. Upon general observation, however, Lyme
borreliosis is by no means a rare disease. It is to be assumed that around one million patients suffer from a manifestation of Lyme borreliosis in the Federal Republic of Germany every year.
Significant characteristics of Lyme borreliosis
Because Lyme borreliosis can affect many organs (so-called multi-organ and multi-system disease), there is a comprehensive differential diagnosis for the often numerous manifestations of the
disease. Observing the pertinent characteristics of LB makes the differential diagnosis easier, and the misidentification of Lyme borreliosis is immensely prevented.
Characteristics of LB
- High risk of infection
(home garden, open nature, pets and wild animals) - Tick bite
- Erythema migrans (EM)
(in 50 % of cases)
(not obligatory) - Lymphocytoma, acrodermatitis chronica atrophicans
(in 3 and 10 % of cases resp.) - Flu-like symptoms incl. without EM
(so-called summer flu) - Fatigue
(exhaustion, feeling of sickness) - Musculoskeletal pains
(e.g. arthritides) - Neurological symptoms (Lyme neuroborreliosis (LNB))
(in about 15 % of cases)
(e.g. polyneuropathy, cranial neuropathy, neural radiculitis and CNS diseases) - Gastrointestinal symptoms
(in 70% of cases) - Eye diseases
- Inner ear disease
- Urogenital pains
- Heart disease
(disrupted stimulus conduction, myocarditis, pericarditis)
Diagnostic strategy
The following situations are significant for the normal practice:
- Fresh tick bite
- Erythema migrans
- Early stage without erythema migrans
- Chronic state (late stage)
Fresh tick bite
Anamnestically, 50-70 % of LB patients deny having a tick bite. A negative tick bite anamnesis also does not rule out LB (173, 174, 175, 176).
If the tick is available, it should be inspected for infectiousness via PCR. However, a negative PCR result does not completely rule out the infection (1). After being bitten by a verifiably infectious tick and a sucking period of about 1-2 hours, the transmission of infection cannot be ruled out, and antibiotic treatment, as in the early stage, must be discussed (see section "Prevention").
Failure to inspect the tick can have the following consequences:
- Observation of the bite area for 4-6 weeks. If reddening (erythema) occurs, or unexplained "flu-like symptoms", immediately consult a physician.
- Immediate serological examination only for tick bite acquired occupationally to cover accident insurance claims, or for patients with history of Lyme borreliosis. (In these cases serological examination is performed to determine initial values for intended continued observation).
Erythema migrans (EM) (early-stage, stage I)
Erythema migrans verifies the disease (LB). There are 2 significant consequences of EM:
- Immediate antibiotic treatment
- Serological examination not required
(exception: relation to occupational accident, accident insurance or patient with LB history)
As antibodies first appear 2-6 weeks after the infection begins (2, 3, 4, 5, 6, 84), it is incorrect and irresponsible to begin antibiotic treatment of EM not immediately, but rather only after determination of a pathological, serological result.
The earlier the antibiotic treatment sets in, the better the infection can be controlled. The chance of treatment is much slimmer 4 weeks after the beginning of the infection (7, 251).
Early antibiotic treatment can prevent the development of antibodies, i.e. no seroconversion takes place. Seronegativity after early antibiotic treatment does not rule out LB, but rather the LB is verified by the EM.
Lymphocytoma is another skin manifestation of the early stage of the disease. Lymphocytoma is an erythematous swelling, primarily around well-circulated areas of the skin (earlobes, nipples, genitalia).
Early stage without Erythema migrans (EM) (stage I)
In 30-50 % of cases, no EM appears in the early stage of Lyme borreliosis (5, 7, 8, 9, 10, 11). The diagnosis must thus be based on other criteria:
- Circumstances of sickness: spending time in home garden and open nature, tick bite
- Symptoms: flu-like symptoms without catarrh around the airways, often with fever, general feeling of sickness, headache, joint and muscle pain, nerve root inflammation
- Extensive physical examination, in particular the skin for purposes of detecting EM, possibly also with diameters below 5 cm (mini-erythema (12)) and lymphocytoma
- Lab diagnosis
Serology - Lymphocyte transformation test (LTT), syn. Lymphocyte proliferation test (LPT)
Cerebral fluid diagnosis
(only with neurological symptoms)
Initial manifestations of Lyme borreliosis sometimes appear weeks or months after the infection begins (4, 13, 117, 124, 200). With corresponding symptoms, the LB must be considered with differential diagnosis. This especially applies to an anamnesis of tick bites or high risk of infection.
Chronic stage (late-stage, stage III)
Chronic Lyme borreliosis is identical to the late-stage manifestation (stage III).
Prior early-stage LB (EM, lymphocytoma, flu-like symptoms) is very important for the diagnosis of the disease in the late stage. However, about 50 % of cases of chronic LB entail no prior or previously known early stage (cf. 4, 7, 13, 117, 124, 200). The lack of an early stage also aids in the misidentification of Lyme borreliosis; regardless, the initial diagnosis of Lyme borreliosis is often significantly delayed (251, 252). The general reason for this is a misdiagnosis, i.e. the assumption of other, non-applicable diseases (252, 253, 254).
The identifying skin manifestation of the late stage is acrodermatitis chronica atrophicans (ACA), which however occurs in only 10 % of cases.
Chronic LB can lead to a number of symptoms (cf. Tab. 3.4). The following manifestations must especially be noted:
- Fatigue (exhaustion, chronic feeling of sickness)
- Encephalopathy (brain capacity disorders)
- Musculoskeletal symptoms
- Neurological symptoms
(neural radiculitis, cranial neuropathy, CNS disease, polyneuropathy) - Gastrointestinal symptoms
- Urogenital symptoms
- Eye symptoms
- Inner ear disease
- Skin symptoms
- Heart diseases
In accordance with the multitude of manifestations, chronic Lyme borreliosis requires extensive general and neurological examination.
Examinations in other areas are often also required, especially for purposes of conducting a variety of technological medical examinations.
Lab diagnosis of chronic Lyme borreliosis entails the following examinations:
- Serological examinations
- Lymphocyte transformation test (LTT)
- Cerebraospinal fluid diagnosis (only for current inflammatory CNS diseases)
Remark concerning occupational illness and accident insurance
Under definite circumstances Lyme disease is acknowledged as occupational disease. The only determining factor is whether the accident (tick bite with transmission of infection), i.e. the infection, occurred while working, namely the patient's proper occupation. For some occupational groups with a high risk of infection (e.g. farmers and forest workers, veterinarians) there is generally an assumed correlation between accident (tick bite) and disease (causal relationship). For other occupational groups this causal relationship must be verified by the patient. In case of a tick bite while on the job, and in the event of subsequent symptoms, it is thus required that the attending physician carefully documents the anamnesis, examination results, and lab results. The patients themselves should record journal entries and render photo documentation of skin changes.
Accident insurance claims also require that the Lyme borreliosis acquired on the job (tick bite with transmission of infection) results in subsequent impairment of the patient's health for a certain period of time. In conjunction with insurance claims, the causal relationship between occupation and infection (accident), as well as the subsequent disease (Lyme borreliosis), must be verified (cf. Chapter 3.1). The occupational illness and subsequent impairment must be verified by the patient (claimant). A proven causal relationship between the tick bite (accident) and occurrence of Lyme borreliosis and continued presence of late-stage Lyme borreliosis must be verified. Verification of current late-stage Lyme borreliosis requires proof of correlation with the accident (tick bite with borrelia infection). General overlapping symptoms between the accident and current disease situation provide a significant instrument for argumentation. It is not uncommon for the tick bite and resulting borrelia infection to be recognised as an occupational illness, however, the subsequent development of late-stage Lyme borreliosis is not acknowledged.
And so it is first necessary to verify the tick bite with transmission of borrelia infection (with likelihood), and also the damage, i.e. persistent late-stage Lyme borreliosis or damage resulting from Lyme borreliosis.
From a legal perspective, the following two aspects must be considered when verifying causality:
- external causation
- internal causation
The external causation concerns the relationship between occupation and infection. The internal causation is that between infection and resulting late-stage Lyme disease.
Symptoms of Lyme borreliosis
Lyme borreliosis is traditionally categorised into 3 stages according to severity and progression:
- Stage I
(early stage with or without EM) - Stage II
(acute Lyme borreliosis) - Stage III
(late stage, chronic progression)
An alternative division of stages can be considered for practical clinical reasons.
Alternative stage categorisation of LB
- Early stage
(EM with or without accompanying symptoms, usually in the first 4 weeks after infection begins, yet sometimes only after months) - Early stage post-dissemination
(EM and multiple organ manifestations after dissemination) - Acute Lyme disease
(acute disease with various organ manifestations as per stage II with traditional stageing, usually some weeks or months after infection begins) - Acute Lyme neuroborreliosis
(acute disease in the nervous system with or without special symptoms of acute Lyme disease) - Chronic Lyme neuroborreliosis
(disease of the central and/or peripheral nervous system in late stage (stage III)), usually in conjunction with other organ manifestations of chronic Lyme borreliosis) - Chronic Lyme borreliosis
(multi-organ and multi-system disease lasting over years or decades with episodes and relatively or entirely painless intervals, including chronic Lyme neuroborreliosis (LNB in 20 % of cases, so not obligatory))
Modern stage categorisation
Recently it has become more common to categorise two stages:
- Early stage
- Late stage
The temporal threshold between these two stages is not clear. The limit of 6 months provided in literature is obsolete. From a practical perspective the early stage lasts about 4 weeks, and afterward the symptoms are to be categorised as late-stage.
Acute Lyme borreliosis and acute Lyme neuroborreliosis (previously stage II) are categorised as early-stage with a certain temporal tolerance.
Early-stage symptoms
The early stage is primarily characterised by the following manifestations:
- Erythema migrans
- Lymphocytoma
- Flu-like accompanying symptoms
- Various organ manifestations after dissemination of the pathogen
For diagnostic, treatment-related, and prognostic reasons, the early stage is temporally defined and is based on the first 4 weeks after the infection begins. As presented above, antibiotic treatment within these 4 weeks is relatively successful. Serological findings after first-time infections appear at the end of this period at the earliest (2, 3, 4, 5, 6, 7, 84).
The early stage is mainly characterised by the disease-verifying EM, although this only occurs in 50-70 % of cases for first-time or new infections (5, 7, 8, 9, 10, 11). It is often accompanied by general flu-like symptoms.
If the pathogens spread throughout the entire organism after infection (inoculation), this dissemination may also lead to diseases of other organs. The thresholds between such a disseminated early stage and acute Lyme disease (stage II) or even a chronic stage (late stage, stage III) are fluid.
The EM may vary significantly in shape, size, and characteristics (4, 8, 9, 12, 13, 117, 124, 200). The traditional shape with margin and central blanching is only present in one-half of cases. That is why in cases of doubt EM should be diagnostically assumed for any erythema regardless of its appearance, especially when the erythema exhibits expansion or persistence over a period of weeks. The CDC (Centers for Disease Control and Prevention) currently note that EM is not present or observed in 30 % of cases, in over 50 % of cases the traditional anulare form is not present and shape variations are common, a macular form is present in 60 % of cases with livid colouration and vesicular changes are visible in the middle (290). It is difficult to demarcate a localised inflammatory reaction around the bite area with a maximum diameter of 2 cm which appears a short time after the tick bite. This inflammation is triggered by a secretion that the tick injects into the skin when biting, and is thus unrelated to borrelia. The demarcation of a so-called mini-erythema migrans with a diameter of less than 5 cm (12) is only possible with continued observation; the EM usually increases in size over time and exhibits a persistence of at least 2 weeks. In case of doubt, a skin biopsy should be conducted for purposes of pathogen verification by PCR.
The initially homogenous erythema can blanche in the middle; all that remains is an arch-shaped margin, and may entail itching and pain with changing characteristics from a span of weeks to many months. On average it heals abruptly after about 10 weeks. With antibiotic treatment the EM usually blanches within several days. Persistence signals inefficient antibiotic treatment and the necessity of switching antibiotics.
Lymphocytoma is equivalent to EM in terms of disease significance and the strategies described. Lymphocytoma entails an inflammatory swelling that can appear in all parts of the body, but especially around well-circulated tissues (earlobes, nipples, scrotum). There are also transitional and overlapping lymphocytoma and EM (so-called atypical EM (14)).
The possibility of a borrelia infection must essentially be assumed with the presence of erythema, of course under consideration of the differential diagnosis. In case of differential diagnostic uncertainties, erythema migrans must be assumed and the corresponding diagnostic and treatment measures taken. In such a situation, dermatological examination is indicated and possibly a skin biopsy for purposes of verifying the pathogen, usually via PCR.
All skin manifestations of Lyme borreliosis in the early and late stage are presented in Tab. 3.5.
Early-stage post-dissemination symptoms
In case of existing erythema or the lack of any skin manifestations, the expansion of the pathogen (dissemination) results in a more or less distinctive disease state. The acute manifestation is referred to as acute Lyme disease (stage II). In less acute courses the early stage may entail numerous organ manifestations (cf. Tab. 3.4) so that there is a seamless transition to the chronic stage. However, there is often a relatively or completely pain-free interval between the early stage and chronic LB (late stage).
Acute Lyme disease (stage II) usually occurs a few weeks or months after the beginning of the infection (inoculation). In accordance with early dissemination, such a disease state may also occur quickly in the presence of existing EM.
The severe disease state often requires inpatient diagnosis and treatment.
The serological findings for acute Lyme disease are generally pathological, although the lack or minor manifestation of the serological findings do not rule out the disease.
With regard to the multi-organ and multi-system disease, acute Lyme borreliosis is not uncommonly associated with nervous system manifestations, i.e. acute Lyme disease may entail acute Lyme neuroborreliosis, or may solely occur as acute neurological symptoms.
Manifestations of Lyme neuroborreliosis
The term "Lyme neuroborreliosis" (LNB) refers to the disease manifestations in the nervous system. It must be noted that Lyme neuroborreliosis is only one of a multitude of organ manifestations. Lyme neuroborreliosis its not an individual disease in and of itself, but rather a partial manifestation of Lyme disease within the entire organism. However, the neurological symptoms may be at the forefront to an extent, or may appear as the only disease phenomenon of Lyme borreliosis.
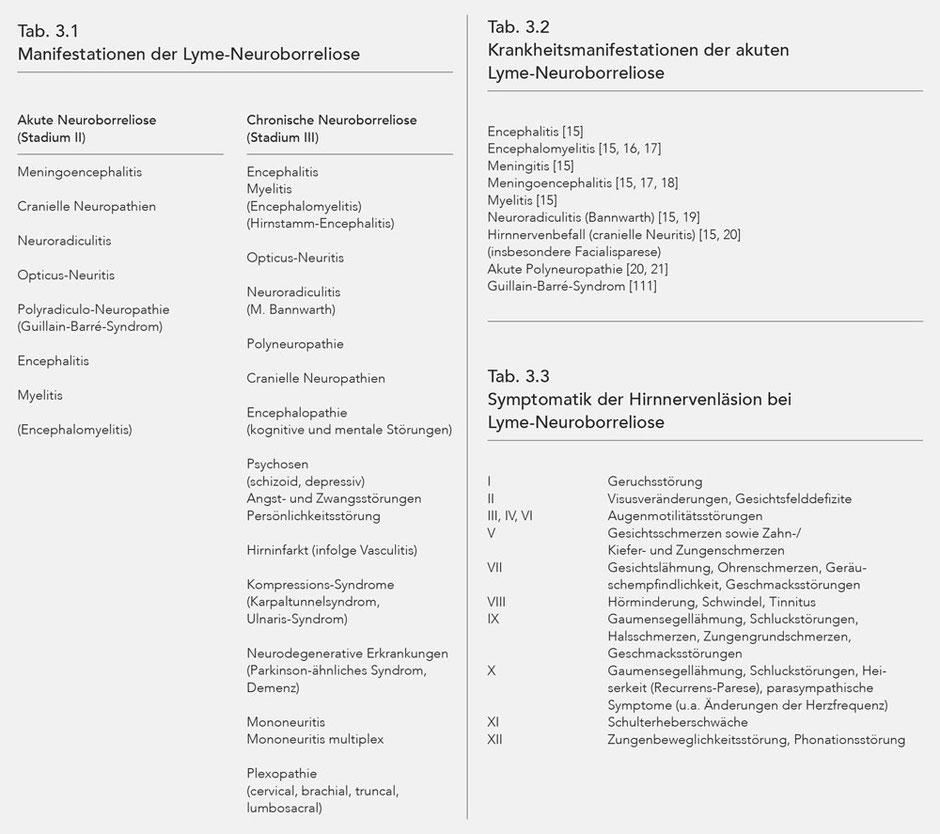
The manifestations of Lyme neuroborreliosis are presented in Table 3.1. The symptoms triggered by borrelia infection are very similar to neurological disease states with other causes. For example, peripheral facial nerve paralysis as part of Lyme borreliosis cannot be phenomenologically differentiated from so-called idiopathic facial nerve paralysis. With regard to the differential diagnostic observation of neurological infectious diseases, overall it can be determined that Borrelia burgdorferi exhibits relatively low virulence compared to other bacteria and some neurotropic viruses. The medical condition of meningitis as a result of infection with Streptococcus pneumoniae is thus more distinctive than is the case with Lyme neuroborreliosis. The same likewise applies to the often similarly severe course of early summer meningoencephalitis (Tick born encephalitis, TBE) which takes a far more dramatic course in terms of manifestation, mortality, and disease consequences than acute Lyme neuroborreliosis. However, Lyme neuroborreliosis may cause severe medical condition with sequelae.
Some symptoms of Lyme neuroborreliosis, however, are more or less typical. This is true for Neuroradiculitis (M. Bannwarth), for isolated temporary or non-progredient myelitis, a chronic form of myelitis with fluctuating manifestation and chronic encephalitis.
All major medical conditions that have special significance for Lyme neuroborreliosis, and [which] may be associated with a problematic differential diagnostic demarcation, are presented below (Tab. 3.1).
Acute Lyme neuroborreliosis
The following neurological symptoms arise from the various localisations of the inflammation in the nervous system (Tab. 3.2).
With cranial neuropathies, failures or functional impairments are usually induced by infectious inflammation in the respective nuclear areas, and so cranial neuropathies are categorised with neural radiculitides for pathological-anatomical purposes. The inflamed lesion may also sometimes occur distally to the subarachnoid space, i.e. peripherally (23). The corresponding impairments are listed below along with the respective cranial nerve number (Tab. 3.3). Refer to the special chapter for further literature.
Chronic Lyme neuroborreliosis
The symptoms of chronic Lyme neuroborreliosis (chronic LNB) largely correspond to the manifestation of acute Lyme neuroborreliosis. However, there are differences when it comes to manifestation
of the disease over time and special characteristics with regard to the brain disorder:
- Progresses in phases of activation and intervals with little or no complaints
- Summarily progressive course
- So-called encephalopathy
- Cerebrovascular symptoms resulting from LB-induced vasculitis
With chronic encephalomyelitis the time-dependent variations go along with various motoric, sensitivity and coordinative disorders in phases of deterioration and intervals with only weak symptoms
(19, 24). The neurological deficits can develop into a progressive condition over time, one which may extend over years.
The phases of deterioration of chronic LNB usually last for weeks or months, and the alleviation of symptoms in the intervals is generally gradual, and for a similar period of time. These relatively long phases of deterioration and only gradual alleviation can allow for a certain degree of differentiation between chronic LNB and multiple sclerosis; however, the differential diagnosis between chronic LNB and MS is often difficult when considering the various technological medical examinations. Pertinent is the consideration of other chronic LB data: disease circumstances, manifestations in various other organs, and so not only in the CNS but also medical findings that indicate chronic LB. Instances in which the symptoms can be attributed to both chronic LNB and multiple sclerosis are rare. The McDonald criteria must be fulfilled in order to diagnose MS.
There is no positive disease marker for both multiple sclerosis and chronic LNB, i.e. there is no technological medical examination, let alone any lab test, that would be able to verify the disease with pathological findings. That is why diagnostic criteria were established for both diseases, which have been defined for MS (most recent revision in 2010) whereas this is not (yet) the case for late-stage (central) Lyme neuroborreliosis. Indeed there are diagnostic criteria for Lyme neuroborreliosis, although these concern (albeit unspokenly) acute Lyme neuroborreliosis and not the chronic manifestations with inflammations in the parenchyma of the central nervous system.
A cerebrovascular disease resulting from vasculitis with LB (17) is also attributed to chronic Lyme neuroborreliosis for practical considerations, although it is primarily a vascular disease. Vasculitis usually results in hemi-symptoms (25, 26) with a protracted and recurrent course.
Encephalopathy with chronic LB and LNB refers to an impairment of cognitive brain performance and mental impairments. Encephalopathy is a very common issue in relation to chronic Lyme borreliosis (27). Cognitive and mental impairments can result in significant disabilities with corresponding effects on social functions (cf. 28, 29, 30).
With encephalopathy the cerebral fluid is usually unremarkable or exhibits only minor changes, especially in the form of increased protein and albumin levels. Such a minor fluid change (blood-liquor barrier) is present in about 5 % of instances of encephalopathy with chronic Lyme borreliosis (28, 31, 32, 33). One frequent manifestation of chronic Lyme neuroborreliosis is chronic peripheral polyneuropathy (20, 21, 34). The lower extremities are mainly affected. This is usually a sensormotoric polyneuropathy of an axonal demyelinating type. The sensory impairment dominates, although motoric impairment may be extensive with the result of severe disability. The cerebral fluid results for chronic polyneuropathy in conjunction with LNB are generally unremarkable (35, 36, 37).
Neurodegenerative diseases (e.g. M. Alzheimer, M. Parkinson, dementia) have been shown in numerous studies to have a pathophysiological relationship with Lyme neuroborreliosis. In light of the constantly increasing neurodegenerative diseases among the aging population, such a relationship could be of significance, although it is not yet apparent to what degree.
Symptoms of chronic Lyme borreliosis
Like the term "early-stage" chronic Lyme disease should also be temporally defined. Disease manifestations of borreliosis that occur 4 weeks after the beginning of infection (inoculation) should be allocated to the condition of chronic Lyme borreliosis (late-stage Lyme disease, LB stage III).
The symptoms of chronic Lyme borreliosis [form] either seamlessly from the early stage, after a pain-free interval of months to years, or primarily form (chronic Lyme borreliosis without preceding early-stage form (4, 7, 13, 117, 124, 200)). The result is that chronic Lyme borreliosis must also be diagnosed if there is no tick bite and no EM if the circumstances, manifestations, and differential diagnosis analysis make it apparent.
Chronic Lyme borreliosis is a persistent infection with vital pathogens. It is not the result of a past infection or a condition after presumably successful antibiotic treatment of the pathogen. Accordingly, numerous studies show that pathogens are cultivated even after highly effective antibiotic treatment (11, 15, 24, 43, 50, 53, 57, 60, 62, 112, 121, 122, 125, 128, 139, 140, 141, 143, 150, 152, 156, 157, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248, 249, 250, 255, 256).
The inflammation of the knee joint (gonitis) is the outlying manifestation in the late-phase (chronic Lyme borreliosis) (43); after differential diagnostic elimination of other causes, gonitis is practically proof of late-phase chronic Lyme borreliosis. However, Lyme arthritis (gonarthritis) only occurs in about 40 % of cases (7). It is thus not at all obligatory for the late-stage manifestation, and lack thereof does not rule out LB.
Because the expansion of borrelia within the organism leads to multi-organ and multi-system diseases, there is an incredibly wide variety of symptoms. The extensive, practically complete set of symptoms of Lyme borreliosis is presented below by organs and organ systems.
The list of such a variety of manifestations also contains the hidden risk of lack of acceptance due to the seeming arbitrariness or lack of differential diagnostic precision. However, all manifestations listed below have been proven in the respective publications, although very rare phenomena (e.g. ALS-like diseases) have only been verified in individual instances.
A comprehensible overview is possible when the symptoms are ordered by most-affected organs and consideration of the general symptoms (Tab. 3.4).
Skin manifestations of LB
Skin manifestations of early- and late-stage LB can be found in Table 3.5 (12, 14, 50, 58, 205-221).
Erythema migrans (EM) and acrodermatitis chronica atrophicans (ACA) verify LB. ACA initially entails an oedematous-infiltrative early phase with livid colouration and swelling. Skin atrophy occurs with advanced progression. Dermatitis atrophicans maculosa is a special form of ACA. Other manifestations are stripe-shaped reddening, "pseudoscleroderma" (morphaea) in the form of ivory-coloured dermatosclerotic plates and juxta-articular fibroid nodes.
Erythema migrans, which verifies the disease, only occurs in about 50 % of cases. EM is not by any means obligatory for the diagnosis of Lyme borreliosis, and its absence does not rule out Lyme borreliosis. Erythema migrans is a visual diagnosis, although the pathogen can often be determined with a skin biopsy by means of PCR (see Chapter 22.17 and 22.18). Histologically, EM is characterised by a perivascular, lymphocytic infiltrate with few plasma cells and isolated eosinophils (cf. Tab. 7.1).
EM may vary significantly in shape and size. Mini-erythemae with a diameter of less than 5 cm are possible, and the pathogen can be verified via PCR. Of special significance is the fact that the erythema can appear in various shapes: anular (en cockade, bull's-eye shape) in about 50 % of cases, macular (more or less consistent reddening) also in about 50 % of cases. Furthermore, the skin around the erythema can exhibit further changes such as papules, vesicles, haemorrhaging, and others. It is not uncommon for the EM to take multiple forms (multiple erythemata migrantia, MEM). Because EM verifies the early stage, it is necessary to consider atypical manifestatons of EM in order to ensure prompt diagnosis and early antibiotic treatment.
In about 3 % of cases lymphocytma occurs in the early stage, and this also verifies the disease. Clinically lymphocytoma occurs as an inflammatory swelling, histologically it is considered a pseudolymphoma designated as borrelia lymphocytoma with reference to the aetiology. Predilection areas are parts of the skin with good blood circulation (e.g. nipples, earlobes, scrotum). Transitions between lymphocytoma and erythema migrans are also possible, and such a combination is categorised as "atypical EM".
Acrodermatitis chronica atrophicans (ACA) occurs in about 10 % of cases of late-stage Lyme borreliosis and verifies the late stage. This is differentiated into an inflammatory stage of the subcutis which progresses into skin atrophy with degeneration of the subcutis. Histologically ACA occurs in the inflammatory stage as a perivascular, lymphocytic infiltrate with isolated plasma cells and few eosinophils. In the atrophic stage there is a mingling of lymphocytic infiltrate with plasma cells, sweltering of the epidermis/dermis/subcutaneous rete ridges, atrophy of the adnexa, and loss of the rete ridges. Refer to Chapter 10.2 for further histological phenomena.

The duration of an erythema migrans can last for weeks or several years (264-285). With antibiotic treatment the erythema migrans abates within days or weeks; the success rate of treatment is 90-95 % (264-270). This equates to a failure rate of 5-10 %. However, there are no studies of collectives concerning persistence issues of EM after antibiotic treatment. All that is known is the failure of antibiotics in a fraction of cases and pathogen verification during skin infection (panniculitis) despite intensive antibiotic treatment (277). Upon failure of antibiotic treatment, persistence of the EM over months or even years is to be expected. The EM may transition into acrodermatitis chronica atrophicans (ACA) in its chronic state (272). The simultaneous presence of EM and ACA (typical late-stage manifestation) implies the potential of a long-term EM (283).
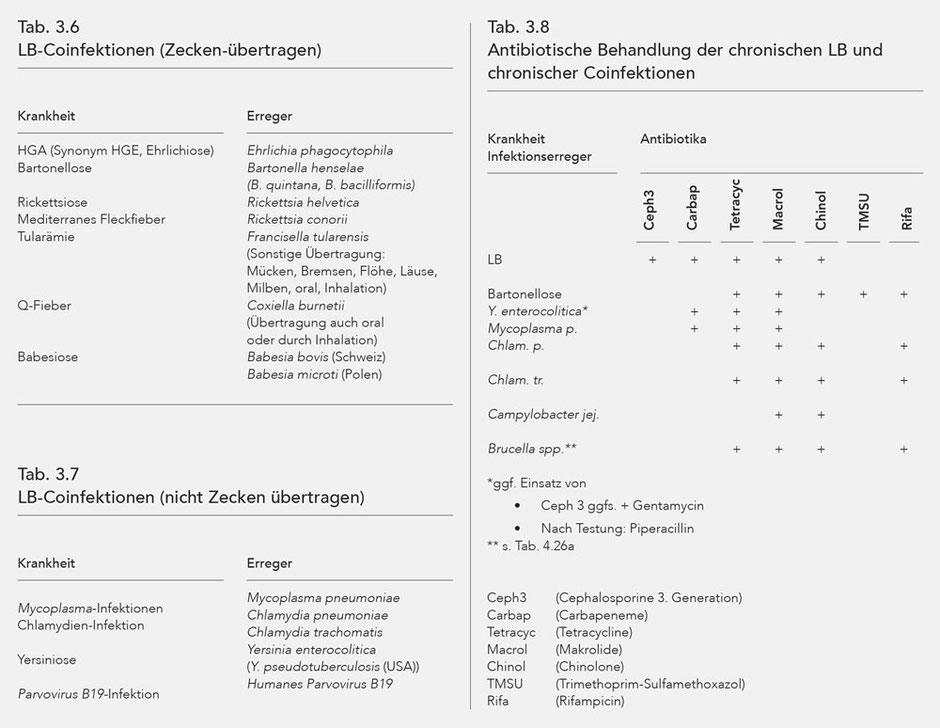
Coinfections
Other infections may be present alongside Lyme borreliosis, the pathological synergy of which either worsens the patient's condition or induces similar manifestations to LB. Such accompanying infections are referred to as coinfections.
Some coinfections, like LB, can be transmitted by ticks, i.e. a tick bite can cause multiple infections. Some coinfections are transmitted without ticks, or there are other ways aside from ticks for them to be transmitted.
The coinfections transmitted by ticks are listed in Table 3.6, and tick-independent coinfections in Table 3.7.
The coinfections make disease manifestations more distinct through modulation of the immune system, and are considered a significant reason for treatment resistance of Lyme borreliosis (188-203).
Bartonella henselae, verified in 40 % of ticks in Europe, is paid special consideration (222).
Bartonella henselae was found in the cerebral fluid of patients with diseases of the central nervous system, and without any prior cat-scratch disease (188). Furthermore, like Bb Bartonella henselae can induce a multi-organ disease (223).
Reactive arthritis

Reactive arthritis is not a nosological entity. Rather it is an umbrella term for disease conditions with post-infectious arthritides. Diagnostic and pathophysiological categorisation is still uncertain. However, the pertinent literature is increasingly providing indications that so-called reactive arthritis is actually a persistent, infectious form of arthritis similar to stage III Lyme arthritis. Accordingly, antibiotic treatment is indicated.
Pathogens that induce reactive arthritis are listed in Tab. 3.9.
Yersiniosis is of particular differential diagnostic significance with regard to LB. Both diseases (LB, reactive arthritis with yersiniosis) typically entail joint inflammation in the lower extremities; the gonitis typical of LB can likewise indicate reactive arthritis with yersiniosis.
An interval of several days to several weeks should be noticed between the symptoms typical of infection and occurrence of reactive arthritis.
Acute reactive arthritis usually regresses spontaneously within 6 months. If this time period is exceeded then this indicates chronic reactive arthritis, which has a reported frequency of 4 - 19 %.
Treatment requires antiphlogistics (NSAR) or anti-TNF compounds.
Timely administration of antibiotics against the underlying infection can prevent the development of reactive arthritis to a degree. Various studies have shown that antibiotics cannot impact the progression of reactive arthritis, although this is solely based on chlamydia infections and a very restrictive antibiotic treatment (204).
Laboratory diagnostics
Laboratory examinations available for diagnostic analysis of Lyme disease are documented in Table 3.10, examinations of cerebrospinal fluid in case of neuroborreliosis in Table 3.11 and further laboratory examinations indicated in various stages of LB in Table 3.12.
Antibodies are verifiable about 2-6 weeks after the infection begins (inoculation) (2, 3, 4, 5, 6, 84, 261). With a previously negative starting value, the appearance of antibodies verifies fresh
infection. In this regard it is prudent to determine a starting value immediately following the tick bite. If antibodies are verifiable at the starting point (as a result of past Bb infection),
the fresh, i.e. new, infection appears in the surge of serological findings (increased titer with ELISA, EIA, greater number of bands in the western blot).
Serological examination entails the quantitative determination of IgM and IgG antibodies as well as the verification of various Borrelia antigens via electrophoretic separation with the western
blot method.
The verification of antibodies is proof of the presence of the infection, but not the disease (LB). Diagnosis of LB is solely based on clinical data: disease conditions, history, reported symptoms, and test results. A positive serological result is merely an indication of a causal relationship between the Borrelia infection and symptoms typical of LB. The serological result can neither verify nor rule out Lyme borreliosis.
Early antibiotic treatment often prevents seroconversion, i.e. the development of antibodies, although the Borrelia infection persists and morphs into chronic Lyme disease (late-stage Lyme disease). Regardless, seronegativity is present in up to 30 % of cases of chronic Lyme borreliosis.
Seronegativity thus does not rule out Lyme borreliosis, i.e. the verification of antibodies is not mandatory for the diagnosis of LB (46-63).
The presence of IgM antibodies is much less common than that of IgG antibodies. The IgM antibody, generally considered an early indicator, occurs in no more than 50 % of early-stage cases (64). In late-stage cases the frequency is only 15 % (65). Much like seronegativity in general, the absence of IgM antibodies does not contradict the diagnosis of a Borrelia infection and subsequent Lyme borreliosis.
The term "seroscar" describes the presence of antibodies after the disease (LB) has abated; this term is only applicable if there are no symptoms of Lyme borreliosis. It is also possible that the serological findings occur in a symptom-free interval period, in which case the relapse of the symptoms (thrust) would exclude the term "seroscar".
The western blot test is more sensitive than the screening tests (49, 66, 291). It is thus prudent to conduct screening tests and western blot simultaneously. Up to a certain point the two procedures both have benefits when it comes to quantification, namely the screening tests for titer levels and the western blot for the number of verifiable bands.
The Borrelia antigens verified with western blot vary in specificity and can also be categorised to different stages to a certain degree. Refer to Table 3.13 for the characteristics (67).
The lymphocyte transformation test (LTT, syn. lymphocyte proliferation test) is based on the measurement of the immunological memory of immune cells (persistent T- and B-memory lymphocytes). The
test was developed in 1979 (68, 69) and first used in relation to Lyme borreliosis in 1981 (70). The diagnostic significance of LTT has since been proven in numerous studies (70-84). Through
methodical development LTT has come to achieve a sensitivity equivalent to that of serological tests (84).
LTT has special significance with regard to seronegative Lyme borreliosis. LTT is positive in about 20 % of seronegative LB patients, and in this situation it is the only indication of a Borrelia infection. Furthermore, positive LTT is a strong indication of current presence of Borrelia within the organism.
As concerns the diagnostic significance of LTT, it is important whether false-positive LTT results are to be expected. This risk is clearly quite low, as shown by the overwhelming number of publications (83, 84).
The T-cell test also examines immunological memory, whereby the emission of cytokines is measured after stimulation of the immune cells with Borrelia antigens. There are currently no durable data for a measurable comparison between LTT and T-cell test.
LTT shows a much higher reactive speed than serological examinations. In the early stage LTT is positive before antibodies are detected. After a sucessful antibiotic treatment LTT becomes negative within 4-6 weeks. With relapse LTT mostly becomes positive again (67).
LTT is thus largely significant for diagnostic problems as a result of ambiguous symptoms, the efficiency test of antibiotic treatment, and monitoring, especially with regard to recurrence (Tab.
3.14).
In a positive serological test the LTT and T-cell test may be negative, and vice versa.
LTT and T-cell test are, as with the serology, merely pertinent to the infection but not to the disease. Negative LTT and T-cell test thus do not rule out LB.
CD57+ NK cells are commonly reduced in case of chronic Lyme borreliosis and Lyme neuroborreliosis (85, 294). The current data do not allow for a definitive assessment.
Lab verification of Lyme borreliosis is based on the verification of Borrelia by culture or polymerase chain reaction (PCR). The cultural cultivation verifies the existence of vital Borrelia; PCR merely documents the genetic material of the borrelia that can theoretically also stem from devitalising microbes or microbe particles. However, from a practical perspective a positive PCR result is also considered pathogenic verification and proof of the presence of Lyme borreliosis.
Various lab tests are conducted for the different stages or disease circumstances. Refer to Table 3.12 for more detailed information.
Cerebro-spinal-fluid (CFS) diagnostics of Lyme neuroborreliosis differentiate between non-specific and specific results. Refer to Table 3.11 for more detailed information. Specific fluid findings verify intrathecal antibodies (formed in the CNS) through titer comparison between serum and liquor with the Reiber method, comparison of the western blot findings in the serum and liquor (number, type of bands), and specification of oligoclonal bands with ELISA. A positive antibody index (AI) indicates intrathecal AB formation, i.e. the AB titer in the liquor is (relatively) increased compared to the serum (considering the natural concentration differences of protein, albumin and IgG in the liquor and serum). Intrathecal antibodies often begin to develop many weeks after the onset of the Borrelia infection in the CNS. In some cases there are no intrathecal antibodies at all in the presence of Lyme neuroborreliosis. Furthermore, intrathecal antibodies may persist many years after regression of the Lyme borreliosis. The intrathecal antibodies' primary diagnostic significance lies in acute Lyme borreliosis, when they occur alongside corresponding symptoms and course of the disease. However, intrathecal antibodies are not proof of existing Lyme borreliosis or Lyme neuroborreliosis in the late stage. On the other hand, the lack of intrathecal antibodies does not by any means rule out stage III Lyme borreliosis, especially as Lyme borreliosis with inflammation of the central nervous system (immunological response by the CNS) only occurs in 5-10 % of cases, i.e. intrathecal antibodies are not to be expected in 90 % of patients with late-stage Lyme borreliosis.
The verification of OspA also verifies the presence of Borrelia burgdorferi s. l. in the CNS [44]. Verification of the pathogen may sometimes occur directly via PCR, and rarely via culture. Certain chemokines, in particular CXCL 13, are likely significant in the determination of the early stage of acute LNB [45]; however, this method is currently not a component of routine diagnosis.
The intrathecal antibodies especially important for the diagnosis of acute LNB are only verifiable about 2 weeks after the onset of neurological symptoms. Furthermore, numerous publications show that intrathecal antibodies are not verifiable in a considerable fraction of cases despite typical clinical manifestation, inflammatory fluid results, and verification of the disease via pathogenic verification (86-105).
Furthermore, it is to be noted that there are usually no pathological liquor results in acute early-stage Lyme neuroborreliosis (LNB), so the diagnosis thereof cannot rely on liquor results. In one study of 799 patients with distinctly acute LNB, liquor changes according to the criteria of the pertinent guidelines (Epidemiological Bulletin of the Robert Koch Institute, Berlin, 38/2007) were present in only 42 cases (5.25 %). The bulletin states, "The lab diagnostic verification of early neuroborreliosis required for the current definition is only fulfilled in a very small number of conveyed neuroborreliosis cases, a problem that has already been noted in a previous report," (171).
Seronegativity in the serum also does not rule out LNB, as revealed by studies of clinically typical cases with pathogenic verification (106-110).
Other technical medical tests

Other testing methods pertaining to the musculoskeletal system, nervous system, heart, and eye are also used in the diagnostic clarification of Lyme borreliosis and Lyme neuroborreliosis. The various methods are listed in Table 3.15. The purpose of the methods is self-explanatory, although some characteristics must be noted: CCT and MRT serve to verify cerebral lesions and expanded, demyelinating clusters whereby these illustrative procedures cannot differentiate between LNB and MS (111-115). SPECT can reveal significant impairment of metabolic activity and circulation in various cerebral regions, including in the presence of encephalopathy (116). The skeletal scintigram serves to verify inflammations, including around the joints, that are highly significant when considering the differential diagnosis for diagnosing chronic Lyme borreliosis.Neuropsychological tests are utilised for encephalopathy; however, cognitive functional impairments are often not recorded while they are verifiable through subjective estimation (with corresponding tests) [28]. An inconspicuous neuropsychological test does not rule out cognitive impairment as a result of encephalopathy with chronic LB. An ophthalmological test can reveal optical neuritis by assessing visual acuity and visual field; these tests are of special significance for retrobulbar optical neuritis, i.e. with normal fundus. VEP (visually evoked potential) allows for the objectification of such a disorder. Furthermore, the ophthalmological test records uveitis anterior, intermedia and posterior (177, 178), vitreitis (179), retinal vasculitis (180), and papilloedema (181, 182). Dermatological testing of skin biopses can histologically and microbiologically reinforce the diagnosis of LB-typical skin change (e.g. with ACA or persistent or recurring EM).
Antibiotic treatment of Lyme borreliosis
Warning
Because there have not yet been any evidence-based studies of the antibiotic treatment of chronic Lyme borreliosis, no guarantee can be made for the following recommended treatments of late-stage (stage III) Lyme borreliosis.
The following 2 facts are of special significance with regard to the efficacy of antibiotic treatment of LB:
- Antibiotic treatment is more effective in the early stage (stage I) than in the late stage (stage III) (7, 251)
- For each antibiotic, treatment success may be delayed or not appear at all, potentially necessitating treatment with another antibiotic (4, 13, 117, 124, 200)
The scientific basis for antibiotic treatment of LB remains insufficient, with the exception of the localised early stage (EM before dissemination). The significant deficits of the clinical-scientific analyses are reflected in the treatment guidelines, the recommendability and evidential basis of which are severely limited in the international literature (4, 13, 117, 118, 124, 200) and which do not meet the medical and healthcare policy requirements.
The following evidential criteria are at the basis of the international recommendations (4, 13, 117, 118, 124, 200):
I At least one randomised study
II At least one well-designed, non-randomised study
III Expert experience based on clinical expertise
The recommendability is based on the evidence level:
A. Good evidence
B. Some evidence
C. Weak evidence
Accordingly, the following levels of recommendability come from the international literature as represented by the IDSA (Infectious Disease Society of America) (118):
Localised early stage A-I
(Amoxicillin for children under the age of 8) B-II
(Cefuroxim) B-III
Disseminated early stage B-II – B-III
(Stage II)
(Independent of type of antibiotic and disease situation)
Late phase B-II – B-III
(Stage III)
When categorising B-III, the expert opinion and clinical experience are equivalent to weak evidence based on non-randomised studies.
The treatment of chronic Lyme borreliosis (late-stage, stage III) is thus not based on a solid foundation, i.e. there are no randomised studies of these important issues.
The recommendations presented here are based on the scientific literature, clinical experience of physicians with expertise in borreliosis, and fundamental research, in particular in the fields of microbiology and immunology. Successful antibiotic treatment is only possible with an efficient immune system. With regard to antibiotic treatment, problems arise from natural or acquired resistance. The pathogen (Bb) can remove itself from the immune system with "escape mechanisms" (164, 183).
The treatment duration is of crucial significance for the efficiency of antibiotic treatment. In the early stage, i.e. the first 4 weeks after inoculation, a failure rate of 10 % is to be expected (119, 120, 121, 122), whereas this number is much higher in chronic manifestations and can be up to 50 % (4, 13, 117, 122, 123, 124, 125, 126, 127, 128, 200).Past works had already referred to the problem areas of chronic Lyme borreliosis and its limited response to treatment (4, 13, 117, 122, 124, 130-135, 200). In all of these studies the treatment duration was limited and usually lasted no longer than 4 weeks. Repeated treatment cycles during such a treatment period also showed considerable treatment failure rates (136, 137, 138).
Chronic Lyme borreliosis is defined as a disease and is precisely presented as such in the literature (139-143, 247, 248, 249, 250, 255, 256). This also corresponds to the fact that after abstaining from adequate antibiotic treatment for weeks or years, the pathogen was verified in various tissues as well as the blood, liquor, and synovial fluid. The clinical course of chronic Lyme borreliosis after untreated erythema migrans was presented in the early works of Steere et al., 1987 (262) and Szer et al., 1991 (263).
Some studies have since become available that verify the positive effect of long-term antibiotic treatment, partially with regard to single aspects (4, 13, 117, 144, 145, 122, 123, 146, 124, 130-135, 147, 148, 126, 127, 128, 149, 147, 139, 136, 137, 150, 151, 152, 141, 138, 153, 142, 143, 200).
The limited effect of antibiotic treatment is revealed in numerous studies: even after presumably highly effective antibiotic treatment, pathogens were cultivated (144, 121, 122, 154, 125, 155, 128, 139, 150, 156, 157). Other factors play a role in vivo under antibiotic treatment (162) that are based on the ability of Borrelia to withdraw from the immune system (167, 122, 159, 160, 161) and to resist antibiotics. After multiple antibiotic treatments (Ceftriaxon, Doxycycline, Cefotaxim) Borrelia could be isolated from the skin (121, 122, 130, 148, 150, 156, 163, 157). A considerable discrepancy between antibiotic sensitivity of the Borrelia in vitro versus in vivo was also verified (125).
The Borrelia's resistance to antibiotics is, among other things, attributed to the intracellular location of the Borrelia (292) and biologically largely inactive eukaryotic shapes (cyst form) (167, 133, 160, 161, 155, 140). Furthermore, a film of complement regulators on the surface of the pathogen was discovered in Borrelia, with the effect of complement resistance with typical "shedding" (159, 160, 161). Other mechanisms, e.g. the diversification of membrane lipoproteins, loss of plasmids, various processes for deactivating complement (167, 160, 161) assist with the "escape mechanism" verified in other bacteria as well, i.e. the ability of the pathogen to withdraw from the immune system. The pathogen's ability to down-regulate proteins (pore-forming protein) could also contribute to the impairment of antibiotic effectiveness (164, 165).
There are 4 randomised studies of chronic Lyme borreliosis that focused on the comparison of various antibiotics and the antibiotic treatment of encephalopathy. These studies primarily showed that Cephalosporins were superior to Penicillin (4, 13, 117, 124, 129, 133, 200). Another study was solely concerned with encephalopathy with Lyme borreliosis (150). Usual dosage of Doxycycline only results in relatively low serum levels and tissue concentrations whereas the concentrations are much higher with the Cephalosporins, i.e. as concerns the minimal inhibitor concentrations (MIC) the values of the Cephalosporins are at least ten times greater than those for Doxycycline (154). Because of these high tissue concentrations, the usage of Cephalosporins to treat chronic Lyme borreliosis is nearly unavoidable. One alternative to this end is Gemifloxacin, which also achieves very high tissue concentrations (184). A very wide treatment spectrum and high tissue concentration of the antibiotics are especially significant with regard to poorly circulated compartments, especially in the extracellular matrix of connective tissue structures (joint capsules, fasciae, tendons), as Borrelia have a particular affinity for such tissues.
Whereas the above mentioned discrepancies concerning the in vitro effect and clinical success were verified for some antibiotics, there is good accordance among the Betalactams, Carbapenems, Macrolides and Tetracyclines. The bactericidal effect is clearly less dependent on the concentration than on the reaction time. The genospecies exhibit variable susceptibility, and the sensitivity may vary within one species. Treatment failure is seen in practically all common antibiotics (125).
Other antibiotics, especially the Carbapenems and Telithromycin, are used clinically although usage thereof is based on test in vitro (125) and there are no clinical studies. The same applies to Tigecycline (185, 186).
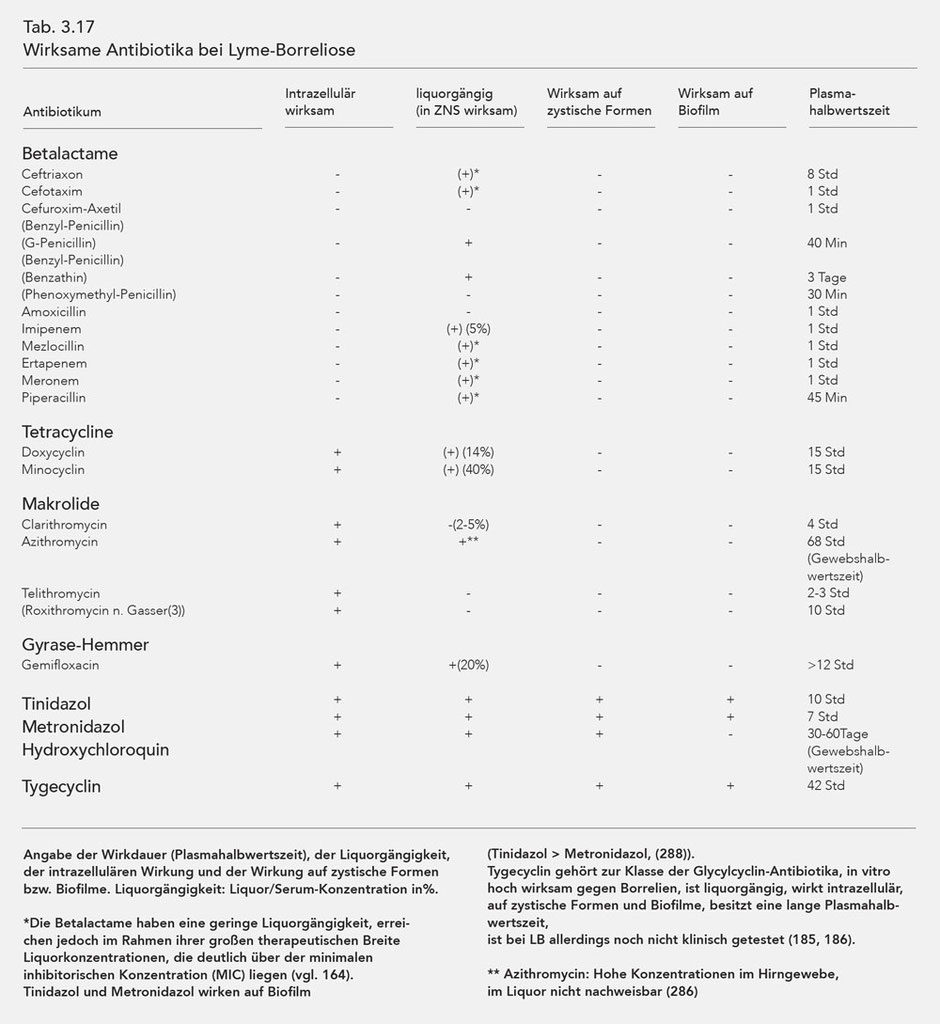
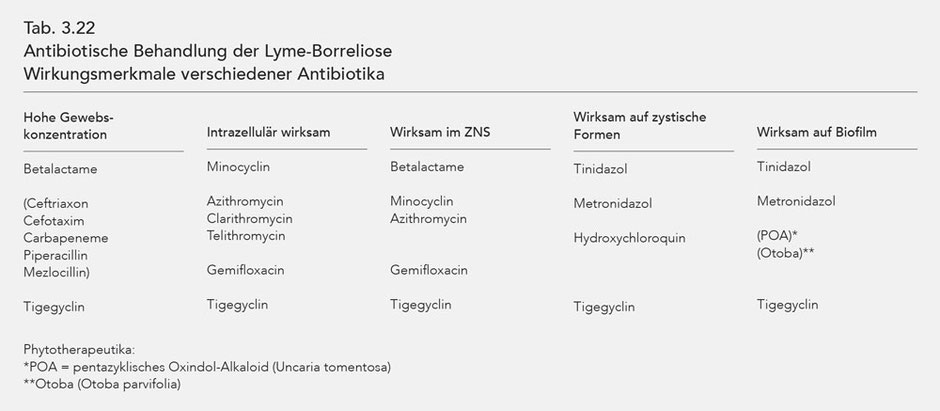
The antibiotics effective against Borrelia are listed in Table 3.17. The intracellular effect, penetration into the cerebro-spinal-fluid (liquor/serum concentration in %), and the effectiveness period (plasma half-life) are also listed.
As explained above, Borrelia are capable of morphologically changing, i.e. forming so-called cystic forms that are resistant to most antibiotics. Normal, spirochaetal Borrelia can reform from the cystic forms in a favourable, especially antibiotic-free environment. As the table shows, only the substances Tinidazol, Metronidazol, and Hydroxychloroquin can influence the cystic forms, whereby Hydroxychloroquin also influences the mobile Borrelia while this is not the case for Metronidazol (140, 166). However, more recent tests show that Tinidazol and Metronidazol are also highly efficient against spirochaetal forms of Borrelia burgdorferi (288). Furthermore Hydroxychloroquin, as a lysomotropic substance, reinforces the effect of macrolides (140) and possibly also that of the tetracyclines.
Another major effect of Tinidazol and Metronidazol is their influence on the biofilm, which was also verified in Borrelia. Both substances proved themselves effective, although Tinidazol was much more effective (Tinidazol differs from Metronidazol with an SO2 molecule) (258, 259, 260).
Antibiotic treatment can either be in the form of monotherapy (Table 3.18) or synchronous combination treatment (Table 3.23 and 3.24).
The efficiency of antibiotic combination treatment has not yet been scientifically proven; this treatment method is based on microbiological findings and not yet systematically examined, empirical data.

Unsuitable antibiotics
A number of antibiotics have been proven to be ineffective in treating Lyme borreliosis (cf. 164) (Table 3.16).
Effective antibiotics
The longer the infection lasts, the more difficult it is to treat Lyme borreliosis with antibiotics. Whereas the early stage before dissemination is susceptible to antibiotics, there are often considerable difficulties with chronic Lyme borreliosis (late stage, stage III).This is not only induced by dissemination, i.e. the spread of the pathogen throughout the entire organism, but is also a result of a progressive adaptation of the pathogen and its resulting ability to withdraw from the immune system and antibiotic effects.
The characteristics of the different antibiotics' effects are listed in Tab. 3.22.
The antibiotics that are effective against Borrelia are listed in Table 3.17 with listing intracellular effect, penetration into the CFS and duration of effectiveness (plasma half-life).
Azithromycin, which accumulates in various organ tissues, has special characteristics. This is especially the case for cerebral tissue. Upon oral application of 500 mg of Azithromycin, cerebral concentrations of about 2.5 µg/g are measured one day later. If one considers the maximum inhibitory concentration (MIC) measured in vitro or the maximum bactericidal concentration (MBC) (125), the cerebral concentration is 100 times that of the MIC and 5 times that of the MBC in vitro (286).
The most recent tests show that the antibiotic Tigecycline is also effective against cystic forms and biofilms (185, 293, 294). The substance is incredibly effective against Borrelia (186), exhibits intracellular effectiveness, possesses good penetration into the liquor and a long tissue half-life (cf. Table 3.17) so that a daily dosage of 50 mg may also suffice for chronic LB and LNB.
Treatment with Roxithromycin is solely recommended by Gasser and Dusleag, 1990 (153). Roxithromycin is not recommended by Hansen et al. based on their study (287) of treatment of Lyme borreliosis.
Monotherapy
Monotherapy, i.e. treatment of LB with only one antibiotic in various stages of the disease, is presented in Table 3.18.
Antibiotic treatment should be adjusted to the patient's weight, especially for children and underweight patients.
Treatment with Cephalosporins of the third generation is also suitable after initially continuous treatment (subsequently) in the form of pulsed treatment; during pulsed treatment the medication is administered 3-4 days out of the week [130].
The administration of third-generation Cephalosporins is also viewed critically by physicians, as they fear aiding intracellular residence and cyst formation of Borrelia burgdorferi [168, 169].
Monitoring the blood count, ALAT, lipase and creatinine is required weekly at first, and then every 2-3 weeks. When using Ceftriaxon, sonographic monitoring of the gall bladder must be performed every 3 weeks to recognise formation of "sludge" in time. When using Macrolides, ECG monitoring is required in two-week intervals.
The risk of a Herxheimer reaction must be noted for any antibiotic treatment of Lyme borreliosis, regardless of the stage. Corticoids should thus only be applied for considerable symptoms (parenteral).
Temporary light to medium worsening of symptoms may occur in the first weeks of antibiotic treatment. A short break in treatment, namely 2 to 3 days, helps to distinguish between drug intolerance and Herxheimer reaction.
Probiotic treatment is necessary to protect the intestinal flora and supports the immune system during long-term antibiotic treatment (e.g. Mutaflor, Omniflora [cf. 224]). In case of severe diarrhea that is hard to treat (e.g. with Saccharomyces boulardii), antibiotic treatment must immediately be interrupted and it must be determined whether a Clostridium difficile infection is present. In case of mycosis in the gastrointestinal tract, antimycotic treatment is required parallel to antibiotic treatment, and for 1-2 weeks beyond the antibiotic treatment. Common antimycotic medications are used for oral and genital mycosis.
Stage-dependent antibiosis
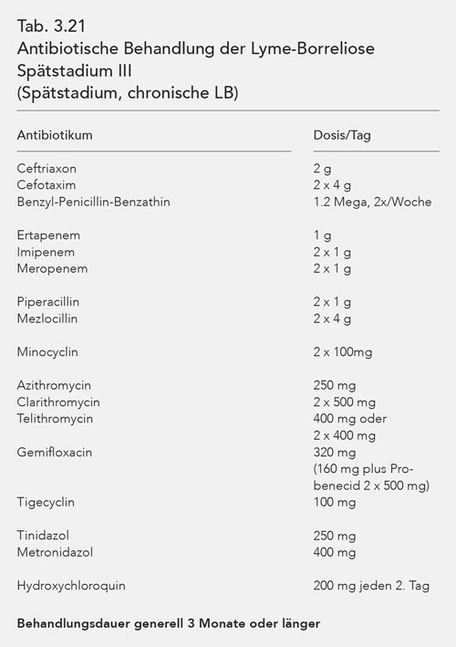
For purposes of a better overview, the antibiotics used in stages I, II and III are listed in tables (Tab. 3.19, 3.20, 3.21). The data of these tables coincide with those of Table 3.18.
To this end it is also noted that synchronous, combined antibiotic treatment is prudent for stage III Lyme borreliosis (cf. Tab. 3.23, 3.24, 3.25).
Combination treatment
Antibiotic treatment of late-stage (stage III) Lyme borreliosis requires antibiotics with specific effectiveness characteristics: high tissue concentration, intracellular effectiveness and
effectiveness in the CNS. Effectiveness against cystic forms and biofilm (Tab. 3.22).

As there are no antibiotics that meet all of these requirements for effective treatment, it is necessary to combine various antibiotics simultaneously and over a sufficient period of time. Stage III Lyme borreliosis thus requires synchronous, combined, long-term antibiotic treatment.
The principle of synchronous, combined antibiotic treatment is described with frequently utilised treatment plans (Tab. 3.23). Along with these examples, a range of other combinations and alternatives is presented in Tab. 3.24.
The antibiotics suitable for synchronous, combined, long-term antibiotic treatment, their dosage and the necessary treatment duration are compiled in the overview in Tab. 3.25.
Essentially, third-generation Cephalosporin should be used with one or two oral antibiotics. If the Cephalosporins are ineffective, the other Betalactams listed in Table 3.25 come into consideration.
Prevention
Because Lyme borreliosis is primarily transmitted by Ixodes ricinus (wood tick) in Europe, the following prevention is based on this carrier. However, personal protection also considers other ticks, especially those found outside of Europe.
Prevention considers the following factors:
- Avoiding tick bites
- Protective clothing
- Repellants
- Examining the skin after exposure
- Removal of biting ticks
With regard to the risk of exposure it must be noted that ticks are found in grasses and shrubbery up to a height of 1 m above the ground. Upon contact the ticks are shed from the surface and reach the skin (beneath the clothes) of all areas of the body. Ticks prefer moisture and warm areas of the skin, although a tick bite can essentially occur anywhere on the body. A special risk arises from contact with wild animals and pets.
This information results in the following primary sources of risk:
- Home garden
- Grass, low shrubbery and similar environments
- Spending time outdoors
- Pets (e.g. horses, dogs, cats)
- Wild animals
Protective gear should prevent penetration by ticks, especially through sealing around the legs and arms. The easiest way to ensure this is to stick the ends of the trouser legs into the socks, or with thick hemming.
Safety suits have also been designed, primarily for children; permethrin is used to impregnate military uniforms.
Various repellants are also available which reduce the risk by spraying directly onto the skin before exposure, although it is not completely effective and the effectiveness is limited to a few hours.
After exposure, i.e. after spending time in open nature, showering is recommended; the body should then be searched for ticks. This is made difficult by the fact that the pre-stages of the adult ticks, the so-called nymphs, are only about 1 mm in size and are thus easy to overlook.
An attached tick must be removed as quickly as possible, as the risk of infection grows the longer the tick is sucking (cf. Chapter 5.2). Only tweezers, tongs, or a sling must be used to remove the tick, as other methods are unsuitable. Once the tick has been grasped with the tweezers or tongs, the tick is slowly and gently removed from the skin, and the bite area is disinfected. Other manipulations around the area of the bite must be avoided.
The benefit of antibiotic prevention after a tick bite correlates to the risk of infection. As this risk is evaluated differently by the studies described in the pertinent literature, there are controversial recommendations as to antibiotic prevention. The work by Nadelmann et al., 2001 concerning one-time administration of Doxycycline for purposes of prevention is particularly significant. In this study it was shown that one-time administration of 200 mg of Doxycycline within 72 hours after the tick bite reduces the risk of infection from 3.2 % to 0.4 %. The authors thus recommended a one-time prophylactic dosage based on these data. Details can be found in Chapter 23.22.
Anhang
General symptoms
- Flue-like symptoms
- Chronic feeling of sickness
- Exhaustion, fatigue
- Reduced ability to withstand stress
- Slight fever, hot flushes, shivering
- Sweating
- Night sweats
- Sore throat
- Headache
- Neck stiffness
- Chest pain, palpitations
- Weight loss
- Weight gain
- Lymph node swelling
- Sexual disorders
- Worsening of symptoms after infection
Nervous system
- Paralysis, Paresis
- Tingling paresthesia
- Sensory loss
- Sense of numbness
- Formication
- Problem with sense of touch
- Muscle spasms
- Involuntary movements
- Tremor
- Mobility disorder
- Carpal tunnel syndrome
- Mononeuritis
- Mononeuritis multiplex
- Plexopathy
- (brachial, lumbosakral)
- Dementia
- (Alzheimer's)
- Amyotropic lateral sclerosis
- Guillain-Barré syndrome
- Tourette's syndrome
- Polyneuropathy
- Nerve root inflammation
- Meningoencephalitis
- Myelitis
- Cerebellitis
- Weakness in the legs
- (Paraparesis)
- Weakness in the arms and hands
Cranial nerves
- Facial paresis
- Optical neuritis
- Visual disorders
- Visual field deficits
- Ocular muscle paresis
- Double vision
- Squinting
- Facial pain
- Numbness, tingling (in the face)
- Smell disorder
- Taste disorder
- Hearing disorder
- Vertigo/loss of balance
- Swallowing disorder
- Tongue movement disorder
- Tinnitus
- Sensitivity to sound
- Pain in tongue, throat
- Hoarseness
- Difficulty lifting the head when lying down
Polyneuropathy
Pain, paresthesia, loss of sensitivity in the feet, legs, hands and arms
Encephalopathy
- Concentration disorder
- Memory loss
- Language loss
- Writing problems
- Reading problems
- Thinking problems
- Depression disorders
- Anxiety
- Behavioural disorders
- Sleep disorders
- Nervousness
- Drowsiness
- Vertigo
Musculoskeletal system
- Joint inflammation
- (Monarthritis)
- (Migratory polyarthritis)
- Joint pains (large and small joints)
- Joint stiffness
- Muscle pain
- Bursitis (e.g. Bursitis suprapatellaris)
- Tendon diseases (tendopathy, enthesiopathy)
- Calcaneal pain
- Shin pain
- Sole pain
- Achilles tendon pain
- Carpal tunnel syndrome
- Epicondylitis
- Back pain
- Chest wall pain
- Spine bone pain
Heart
- Heart muscle inflammation (myocarditis)
- Pericarditis
- Heart enlargement
- (dilatative cardiomyopathy)
- Cardiac arrhythmia
- Conduction disorders
Skin
- Erythema migrans (EM)
- Recurring EM
- Multiple EM
- Acrodermatitis chronica atrophicans (ACA) (early and late stage)
- Fibroma
- Papulosquamous lesions
- Morphaea (localised, pale, hardened skin lesions)
- Tingling or burning pain in the skin
- Itching
Eye
- Eye inflammation (uveitis)
- Outer eye inflammation (scleritis, episcleritis)
- Retinal vasculitis
- Neural ophthalmological disorders
- Eye muscle paresis
- Eye muscle inflammation (ocular myositis)
- Conjunctivitis
- Iritis
- Retinitis
- Vitreitis
- Cornea oedema
- Exudative ablatio retinae
- Retinal vasculitis
- Macular oedema
- Papillitis
- Iridocyclitis
Gastrointestinal symptoms
- Dyspeptic syndrome
- Queasiness
- Diarrhea
- Stomach pain
- Abdominal pain
- Constipation
Urogenital system
- Hyperreflexia of the detrusor (polyuria, frequent urination)
- Urinary retention
- Incontinence
- Nycturia
- Interstitial cystitis
Other symptoms
- Jaw pain
- Sinusitis
Other
- Vasculitis (esp. cerebral infarction)
- References
-
- Fingerle V, Wilske B. Abschlußbericht zur im Jahr 2004 durchgeführten Studie „Epidemiologische Aspekte zeckenübertragener Erkrankungen in Bayern: Lyme-Borreliose“ im Rahmen der „Gesundheitsinitiative: Bayern aktiv“. Bayerisches Staatsministerium für Umwelt, Gesundheit und Verbraucherschutz; München Dez. 2005.
- Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 1993; 167(2):392-400.
- Molloy PJ, Berardi VP, Persing DH, Sigal LH. Detection of multiple reactive protein species by immunoblotting after recombinant outer surface protein A lyme disease vaccination. Clin Infect Dis 2000; 31(1):42-7.
- Steere AC. Lyme disease. N Engl J Med 1989; 321(9):586-96.
- Satz N. Klinik der Lyme-Borreliose. Verlag Hans Huber, 2002.
- von Baehr V, Liebenthal C, Gaida B, Schmidt FP, von Baehr R, Volk HD. Untersuchungen zur diagnostischen Wertigkeit des Lymphozytentransformationstestes bei Patienten mit Borreliose. J Lab Med 2007; 31(3):149-158.
- Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol 1994; 21(3):454-61.
- Meek JI, Roberts CL, Smith EV Jr, Cartter ML. Underreporting of Lyme disease by Connecticut physicians, 1992. J Public Health Manag Pract 1996; 2(4):61-5.
- Boltri JM, Hash RB, Vogel RL. Patterns of Lyme disease diagnosis and treatment by family physicians in a southeastern state. J Community Health 2002; 27(6):395-402.
- Cameron D, Gaito A, Harris N, Bach G, Bellovin S, Bock K, Bock S, Burrascano J, Dickey C, Horowitz R, Phillips S, Meer-Scherrer L, Raxlen B, Sherr V, Smith H, Smith P, Stricker R; ILADS Working Group. Evidence-based guidelines for the management of Lyme disease. Expert Rev Anti Infect Ther 2004; 2(1 Suppl):S1-13.
- Sigal LH. Treatment of Lyme Disease. UpToDate, 2006.
- Weber K, Wilske B. Mini erythema migrans--a sign of early Lyme borreliosis. Dermatology 2006; 212(2):113-6.
- Steere AC, Dhar A, Hernandez J, Fischer PA, Sikand VK, Schoen RT, Nowakowski J, McHugh G, Persing DH. Systemic symptoms without erythema migrans as the presenting picture of early Lyme disease. Am J Med 2003; 114(1):58-62.
- Hassler D. Langzeitbeobachtungen zum Krankheitsbild der Lyme-Borreliose in einem Endemiegebiet. Habilitationsschrift Universität Erlangen, 1997.
- Halperin JJ, Luft BJ, Anand AK, Roque CT, Alvarez O, Volkman DJ, Dattwyler RJ. Lyme neuroborreliosis: central nervous system manifestations. Neurology 1989; 39(6):753-9.
- Drouet A, Meyer X, Guilloton L, Mullet JP, Dusseau JY, Denoyel GA, Felten D. [Acute severe leukoencephalitis with posterior lesions due to Borrelia burgdorferi infection]. [Article in French]. Presse Med 2003; 32(34):1607-9.
- Pfister HW, Wilske B, Weber K. Lyme borreliosis: basic science and clinical aspects. Lancet 1994; 343(8904):1013-6.
- Bertrand E, Szpak GM, Piłkowska E, Habib N, Lipczyńska-Lojkowska W, Rudnicka A, Tylewska-Wierzbanowska S, Kulczycki J.Central nervous system infection caused by Borrelia burgdorferi. Clinico-pathological correlation of three post-mortem cases. Folia Neuropathol 1999; 37(1):43-51.
- Pfister HW, Einhäupl KM, Wilske B, Preac-Mursic V. Bannwarth’s syndrome and the enlarged neurological spectrum of arthropod-borne borreliosis. Zentralbl Bakteriol Mikrobiol Hyg A 1987; 263(3):343-7.
- Halperin JJ. Lyme disease and the peripheral nervous system. Muscle Nerve 2003; 28(2):133-43.
- Kristoferitsch W. Neuropathien bei Lyme-Borreliose. Springer Verlag Wien/New York, 1989.
- Ljøstad U, Skogvoll E, Eikeland R, Midgard R, Skarpaas T, Berg A, Mygland A. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol 2008; 7(8):690-5.
- Tokunaga H, Ohyagi Y, Furuya H, Araki T, Yamada T, Isogai E, Kira J. [A patient with neuroborreliosis presenting gadolinium-enhanced MRI lesions in bilateral facial nerves]. [Article in Japanese]. Rinsho Shinkeigaku 2001; 41(9):632-4.
- Lawrence C, Lipton RB, Lowy FD, Coyle PK. Seronegative chronic relapsing neuroborreliosis. Eur Neurol 1995; 35(2):113-7.
- Rénard C, Marignier S, Gillet Y, Roure-Sobas C, Guibaud L, Des Portes V, Lion-François L. [Acute hemiparesis revealing a neuroborreliosis in a child]. [Article in French]. Arch Pediatr 2008; 15(1):41-4.
- Klingebiel R, Benndorf G, Schmitt M, von Moers A, Lehmann R. Large cerebral vessel occlusive disease in Lyme neuroborreliosis. Neuropediatrics 2002; 33(1):37-40.
- Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, Katz JN, Liang MH. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 1994; 121(8):560-7.
- Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT, Evans J, Weinstein A, Schmid CH, Klempner MS. Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology 2003; 60(12):1916-22.
- McAuliffe P, Brassard MR, Fallon B. Memory and executive functions in adolescents with posttreatment Lyme disease. Appl Neuropsychol 2008; 15(3):208-19.
- Bloom BJ, Wyckoff PM, Meissner HC, Steere AC. Neurocognitive abnormalities in children after classic manifestations of Lyme disease. Pediatr Infect Dis J 1998; 17(3):189-96.
- Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, Dattwyler R, Chandler B. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 2003; 60(12):1923-30.
- Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, Slavov I, Cheng J, Dobkin J, Nelson DR, Sackeim HA. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008; 70(13):992-1003.
- Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A.Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 2001; 345(2):85-92.
- Kindstrand E, Nilsson BY, Hovmark A, Nennesmo I, Pirskanen R, Solders G, Asbrink E.Polyneuropathy in late Lyme borreliosis - a clinical, neurophysiological and morphological description. Acta Neurol Scand 2000; 101(1):47-52.
- Kristoferitsch W, Lanschützer H. [Oligoclonal immunoglobulin M in the cerebrospinal fluid of patients with Garin-Bujadoux-Bannwarth meningopolyneuritis]. [Article in German]. Wien Klin Wochenschr 1986; 98(12):386-8.
- Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, Dattwyler RJ. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 1992; 267(10):1364-7.
- Steere AC, Berardi VP, Weeks KE, Logigian EL, Ackermann R. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis 1990; 161(6):1203-9.
- Miklossy J, Kasas S, Zurn AD, McCall S, Yu S, McGeer PL. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J Neuroinflammation 2008; 5:40.
- Nicolson GL. Chronic Bacterial and Viral Infections in Neurodegenerative and Neurobehavioral Diseases. Lab Med 2008; 39(5):291-299.
- Almeida OP, Lautenschlager NT. Dementia associated with infectious diseases. Int Psychogeriatr 2005; 17 Suppl 1:S65-77.
- Cassarino DS, Quezado MM, Ghatak NR, Duray PH. Lyme-associated parkinsonism: a neuropathologic case study and review of the literature. Arch Pathol Lab Med 2003; 127(9):1204-6.
- McDonald AB. Alzheimer’s disease Braak Stage progressions: reexamined and redefined as Borrelia infection transmission through neural circuits. Med Hypotheses 2007; 68(5):1059-64.
- Steere AC, Hutchinson GJ, Rahn DW, Sigal LH, Craft JE, DeSanna ET, Malawista SE. Treatment of the early manifestations of Lyme disease. Ann Intern Med 1983; 99(1):22-6.
- Coyle PK, Schutzer SE, Deng Z, Krupp LB, Belman AL, Benach JL, Luft BJ.Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 1995; 45(11):2010-5.
- Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, Koedel U. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun 2007; 75(9):4351-6.
- Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis 2001; 33(6):780-5.
- Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A.Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 2001; 345(2):85-92.
- Dejmková H, Hulínska D, Tegzová D, Pavelka K, Gatterová J, Vavrík P. Seronegative Lyme arthritis caused by Borrelia garinii. Clin Rheumatol 2002; 21(4):330-4.
- Tylewska-Wierzbanowska S, Chmielewski T. Limitation of serological testing for Lyme borreliosis: evaluation of ELISA and western blot in comparison with PCR and culture methods. Wien Klin Wochenschr 2002; 114(13-14):601-5.
- Breier F, Khanakah G, Stanek G. Kunz G, Aberer E, Schmidt B, Tappeiner G. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br J Dermatol 2001; 144(2):387-92.
- Wang P, Hilton E. Contribution of HLA alleles in the regulation of antibody production in Lyme disease. Front Biosci 2001; 6:B10-6.
- Grignolo MC, Buffrini L, Monteforte P, Rovetta G. [Reliability of a polymerase chain reaction (PCR) technique in the diagnosis of Lyme borreliosis]. [Article in Italian]. Minerva Med 2001; 92(1):29-33.
- Honegr K, Hulínská D, Dostál V. Gebouský P, Hanková E, Horácek J, Vyslouzil L, Havlasová J. [Persistence of Borrelia burgdorferi sensu lato in patients with Lyme borreliosis]. [Article in Czech]. Epidemiol Mikrobiol Imunol 2001; 50(1):10-6.
- Eldøen G, Vik IS, Vik E, Midgard R. [Lyme neuroborreliosis in More and Romsdal]. [Article in Norwegian]. Tidsskr Nor Laegeforen 2001; 121(17): 2008-11.
- Wilke M, Eiffert H, Christen HJ, Hanefeld F. Primarily chronic and cerebrovascular course of Lyme neuroborreliosis: case reports and literature review. Arch Dis Child 2000; 83(1):67-71.
- Bertrand E, Szpak GM, Piłkowska E, Habib N, Lipczyńska-Lojkowska W, Rudnicka A, Tylewska-Wierzbanowska S, Kulczycki J. Central nervous system infection caused by Borrelia burgdorferi. Clinico-pathological correlation of three post-mortem cases. Folia Neuropathol 1999; 37(1):43-51.
- Oksi J, Marjamäki M, Nikoskelainen J, Viljanen MK. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann Med 1999; 31(3):225-32.
- Aberer E, Kersten A, Klade H, Poitschek C, Jurecka W. Heterogeneity of Borrelia burgdorferi in the skin. Am J Dermatopathol 1996; 18(6):571-9.
- Luft BJ, Dattwyler RJ, Johnson RC, Luger SW, Bosler EM, Rahn DW, Masters EJ, Grunwaldt E, Gadgil SD. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann Intern Med 1996; 124(9):785-91.
- Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection 1996; 24(3):218-26.
- Coyle PK, Schutzer SE, Deng Z, Krupp LB, Belman AL, Benach JL, Luft BJ. Author Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 1995; 45(11):2010-5.
- Häupl T, Hahn G, Rittig M, Krause A, Schoerner C, Schönherr U, Kalden JR, Burmester GR. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum 1993; 36(11):1621-6.
- Nadelman RB, Pavia CS, Magnarelli LA, Wormser GP. Isolation of Borrelia burgdorferi from the blood of seven patients with Lyme disease. Am J Med 1990; 88(1):21-6.
- Lomholt H, Lebech AM, Hansen K, Brandrup F, Halkier-Sørensen L. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Derm Venereol 2000; 80(5):362-6.
- Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC.Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis 2001; 33(6):780-5.
- Engstrom SM, Shoop E, Johnson RC. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol 1995; 33(2):419-27.
- Von Baehr V. Die Labordiagnostik der Borrelieninfektion. umwelt-medizin-gesellschaft, 2/2009.
- Dwyer JM, Johnson C, Desaules M. Behaviour of human immunoregulatory cells in culture. I. Variables requiring consideration for clinical studies. Clin Exp Immunol 1979; 38(3):499-513.
- Johnson C, Dwyer JM. Quantitation of spontaneous suppressor cell activity in human cord blood lymphocytes and their behavior in culture (abstract). Fed Proc 1981; 40:1075.
- Sigal LH, Moffat CM, Steere AC, Dwyer JM. Cellular immune findings in Lyme disease. Yale J Biol Med 1984; 57(4):595-8.
- Dattwyler RJ, Thomas JA, Benach JL, Golightly MG. Cellular immune response in Lyme disease: the response to mitogens, live Borrelia burgdorferi, NK cell function and lymphocyte subsets. Zentralbl Bakteriol Mikrobiol Hyg A 1986; 263(1-2):151-9.
- Sigal LH, Steere AC, Freeman DH, Dwyer JM. Proliferative responses of mononuclear cells in Lyme disease. Reactivity to Borrelia burgdorferi antigens is greater in joint fluid than in blood. Arthritis Rheum 1986; 29(6):761-9.
- Dattwyler RJ, Volkman DJ, Luft BJ, Halperin JJ, Thomas J, Golightly MG. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med 1988; 319(22):1441-6.
- Dressler F, Yoshinari NH, Steere AC. The T-cell proliferative assay in the diagnosis of Lyme disease. Ann Intern Med 1991; 115(7):533-9.
- Krause A, Brade V, Schoerner C, Solbach W, Kalden JR, Burmester GR. T cell proliferation induced by Borrelia burgdorferi in patients with Lyme borreliosis. Autologous serum required for optimum stimulation. Arthritis Rheum 1991; 34(4):393-402.
- Schempp C, Owsianowski M, Lange R, Gollnick H. Comparison of Borrelia burgdorferi ultrasonicate and whole B, burgdorferi cells as a stimulus for T-cell proliferation and GM-CSF secretion in vitro. Zentralbl Bakteriol 1993; 279(3):417-25.
- Buechner SA, Lautenschlager S, Itin P, Bircher A, Erb P. Lymphoproliferative responses to Borrelia burgdorferi in patients with erythema migrans, acrodermatitis chronica atrophicans, lymphadenosis benigna cutis, and morphea. Arch Dermatol 1995; 131(6):673-7.
- Breier P, Klade H, Stanek G, Poitschek C. Poitschek C, Kirnbauer R, Dorda W, Aberer E. Lymphoproliferative responses to Borrelia burgdorferi in circumscribed scleroderma. Br J Dermatol 1996; 134(2):285-91.
- Huppertz HI, Mösbauer S, Busch DH, Karch H. Lymphoproliferative responses to Borrelia burgdorferi in the diagnosis of Lyme arthritis in children and adolescents. Eur J Pediatr 1996; 155(4):297-302.
- Rutkowski S, Busch DH, Huppertz HI. Lymphocyte proliferation assay in response to Borrelia burgdorferi in patients with Lyme arthritis: analysis of lymphocyte subsets. Rheumatol Int 1997; 17(4):151-8.
- Pohl-Koppe A, Kaunicnik A, Wilske B. Characterization of the cellular and humoral immune response to outer surface protein C and outer surface protein 17 in children with early disseminated Lyme borreliosis. Med Microbiol Immunol 2001; 189(4):193-200.
- Vaz A, Glickstein L, Field JA, McHugh G, Sikand VK, Damle N, Steere AC. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun 2001; 69(12):7437-44.
- Valentine-Thon E, Ilsemann K, Sandkamp M. A novel lymphocyte transformation test (LTT-MELISA) for Lyme borreliosis. Diagn Microbiol Infect Dis 2007; 57(1):27-34.
- von Baehr V, Doebis C, Volk HD, von Baehr R. The lymphocyte transformation test for borrelia detects active lyme borreliosis and verifies effective antibiotic treatment. Open Neurol J. 2012; 6:104-12.
- Stricker RB, Winger EE. Decreased CD57 lymphocyte subset in patients with chronic Lyme disease. Immunol Lett 2001; 76(1):43-8.
- Zbinden R, Stech J, Bürgi W, Meier T. [Demonstration of intrathecal antibody formation against Borrelia burgdorferi in Lyme neuroborreliosis]. [Article in German]. Schweiz Med Wochenschr 1993;123(48):2293-8.
- Kaiser R, Lücking CH. Intrathecal synthesis of specific antibodies in neuroborreliosis. Comparison of different ELISA techniques and calculation methods. J Neurol Sci 1993; 118(1):64-72.
- Hammers-Berggren S, Hansen K, Lebech AM, Karlsson M. Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: a follow-up study. Neurology 1993; 43(1):169-75.
- Hansen K, Lebech AM. The clinical and epidemiological profile of Lyme neuroborreliosis in Denmark 1985-1990. A prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody production. Brain 1992; 115(Pt 2):399-423.
- Blanc F, Jaulhac B, Fleury M, de Seze J, de Martino SJ, Remy V, Blaison G, Hansmann Y, Christmann D, Tranchant C. Relevance of the antibody index to diagnose Lyme neuroborreliosis among seropositive patients. Neurology 2007; 69(10):953-8.
- Bednárová J, Stourac P, Adam P. Relevance of immunological variables in neuroborreliosis and multiple sclerosis. Acta Neurol Scand 2005; 112(2):97-102.
- Kaiser R. False-negative serology in patients with neuroborreliosis and the value of employing of different borrelial strains in serological assays. J Med Microbiol 2000; 49(10):911-5.
- Oschmann P, Wellensiek HJ, Zhong W, Dorndorf W, Pflughaupt KW. Relationship between the Borrelia burgdorferi specific immune response and different stages and syndromes in neuroborreliosis. Infection 1997; 25(5):292-7.
- Kaiser R, Rauer S. Analysis of the intrathecal immune response in neuroborreliosis to a sonicate antigen and three recombinant antigens of Borrelia burgdorferi sensu stricto. Eur J Clin Microbiol Infect Dis 1998; 17(3):159-66.
- Treib J, Woessner R, Dobler G, Fernandez A, Holzer G, Schimrigk K. Clinical value of specific intrathecal production of antibodies. Acta Virol 1997; 41(1):27-30.
- Wilske B, Bader L, Pfister HW, Preac-Mursic V. [Diagnosis of Lyme neuroborreliosis. Detection of intrathecal antibody formation]. [Article in German]. Fortschr Med. 1991; 109(22):441-6.
- Hansen K, Lebech AM. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi--specific immunoglobulin G, A, and M. Ann Neurol 1991; 30(2):197-205.
- Kaiser R, Rasiah C, Gassmann G, Vogt A, Lücking CH. Intrathecal antibody synthesis in Lyme neuroborreliosis: use of recombinant p41 and a 14-kDa flagellin fragment in ELISA. J Med Microbiol 1993; 39(4):290-7.
- Tumani H, Nölker G, Reiber H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology 1995; 45(9):1663-70.
- Woessner R, Treib J, Haass A, Stoll M, Holzer G, Schimrigk K. [Value of antibody titers for diagnosis of neuroborreliosis]. [Article in German]. Nervenarzt 1998; 69(8):694-7.
- Hansen K, Cruz M, Link H. Oligoclonal Borrelia burgdorferi-specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J Infect Dis 1990; 161(6):1194-202.
- Halperin JJ, Luft BJ, Anand AK, Roque CT, Alvarez O, Volkman DJ, Dattwyler RJ. Lyme neuroborreliosis: central nervous system manifestations. Neurology 1989; 39(6):753-9.
- Tumani H, Nölker G, Reiber H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology 1995; 45(9):1663-70.
- Wilske B, Schierz G, Preac-Mursic V, von Busch K, Kühbeck R, Pfister HW, Einhäupl K.Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth’s syndrome). J Infect Dis 1986; 153(2):304-14.
- Halperin JJ, Volkman DJ, Wu P. Central nervous system abnormalities in Lyme neuroborreliosis. Neurology 1991; 41(10):1571-82.
- Eldøen G, Vik IS, Vik E, Midgard R. [Lyme neuroborreliosis in More and Romsdal]. [Article in Norwegian]. Tidsskr Nor Laegeforen 2001; 121(17):2008-11.
- Wilke M, Eiffert H, Christen HJ, Hanefeld F. Primarily chronic and cerebrovascular course of Lyme neuroborreliosis: case reports and literature review. Arch Dis Child 2000; 83(1):67-71.
- Bertrand E, Szpak GM, Piłkowska E, Habib N, Lipczyńska-Lojkowska W, Rudnicka A, Tylewska-Wierzbanowska S, Kulczycki J. Central nervous system infection caused by Borrelia burgdorferi. Clinico-pathological correlation of three post-mortem cases. Folia Neuropathol 1999 ;37(1):43-51.
- Keller TL, Halperin JJ, Whitman M. PCR detection of Borrelia burgdorferi DNA in cerebrospinal fluid of Lyme neuroborreliosis patients. Neurology 1992; 42(1):32-42.
- Lawrence C, Lipton RB, Lowy FD, Coyle PK. Seronegative chronic relapsing neuroborreliosis. Eur Neurol 1995; 35(2):113-7.
- Victor M und Ropper AH. Principles of Neurology. McGraw-Hill, 2001.
- Oksi J, Kalimo H, Marttila RJ, Marjamäki M, Sonninen P, Nikoskelainen J, Viljanen MK.Inflammatory brain changes in Lyme borreliosis. A report on three patients and review of literature. Brain 1996; 119(Pt 6):2143-54.
- Bertrand E, Szpak GM, Piłkowska E, Habib N, Lipczyńska-Lojkowska W, Rudnicka A, Tylewska-Wierzbanowska S, Kulczycki J. Central nervous system infection caused by Borrelia burgdorferi. Clinico-pathological correlation of three post-mortem cases. Folia Neuropathol 1999; 37(1):43-51.
- Pincemaille O, Pin I, Wroblewski I, François P, Gratacap B, Joannard A, Bost M. [Meningo-encephalomyelitis in Lyme disease]. [Article in French]. Arch Fr Pediatr 1990; 47(1):39-41.
- Kobayashi K, Mizukoshi C, Aoki T, Muramori F, Hayashi M, Miyazu K, Koshino Y, Ohta M, Nakanishi I, Yamaguchi N. Borrelia burgdorferi-seropositive chronic encephalomyelopathy: Lyme neuroborreliosis? An autopsied report. Dement Geriatr Cogn Disord 1997; 8(6):384-90.
- Logigian EL, Kaplan RF, Steere AC. Successful treatment of Lyme encephalopathy with intravenous ceftriaxone. J Infect Dis 1999; 180(2):377-83.
- Steere AC. Lyme disease. N Engl J Med 2001; 345(2):115-25.
- IDSA practice guidelines for the treatment of Lyme disease. UpToDate 2006.
- Steere AC. Seronegative Lyme disease. JAMA 1993; 270(11):1369.
- Weber K, Preac-Mursic V, Wilske B, Thurmayr R, Neubert U, Scherwitz C. A randomized trial of ceftriaxone versus oral penicillin for the treatment of early European Lyme borreliosis. Infection 1990; 18(2):91-6.
- Strle F, Preac-Mursic V, Cimperman J, Ruzic E, Maraspin V, Jereb M. Azithromycin versus doxycycline for treatment of erythema migrans: clinical and microbiological findings. Infection 1993; 21(2):83-8.
- Preac-Mursic V, Weber K, Pfister HW, Wilske B, Gross B, Baumann A, Prokop J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 1989; 17(6):355-9.
- Gasser R, Reisinger E, Eber B, Pokan R, Seinost G, Berglöff J, Horwarth R, Sedaj B, Klein W. Cases of Lyme borreliosis resistant to conventional treatment: improved symptoms with cephalosporin plus specific beta-lactamase inhibition Microb Drug Resist 1995; 1(4):341-4.
- Dattwyler RJ, Halperin JJ, Volkman DJ, Luft BJ. Treatment of late Lyme borreliosis--randomised comparison of ceftriaxone and penicillin. Lancet 1988; 1(8596):1191-4.
- Hunfeld KP, Ruzic-Sabljic E, Norris DE, Kraiczy P, Strle F. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrob Agents Chemother 2005; 49(4):1294-301.
- Dattwyler RJ, Halperin JJ, Pass H, Luft BJ. Ceftriaxone as effective therapy in refractory Lyme disease. J Infect Dis 1987; 155(6):1322-5.
- Manning PG. Fulminant refractory Lyme disease. Iowa Med 1989; 79(6):277-80.
- Phillips SE, Mattman LH, Hulínská D, Moayad H. A proposal for the reliable culture of Borrelia burgdorferi from patients with chronic Lyme disease, even from those previously aggressively treated. Infection 1998; 26(6):364-7.
- Hassler D, Zöller L, Haude M, Hufnagel HD, Heinrich F, Sonntag HG. Cefotaxime versus penicillin in the late stage of Lyme disease--prospective, randomized therapeutic study. Infection 1990; 18(1):16-20.
- Hassler D, Riedel K, Zorn J, Preac-Mursic V. Pulsed high-dose cefotaxime therapy in refractory Lyme borreliosis. Lancet 1991; 338(8760):193.
- Hassler D. Cefotaxim in der Behandlung der chronischen Lyme-Borreliose. Fortschr Antimicr Antineopl Chemother 1992; 11:109-118.
- Hassler D, Maiwald M. [Reinfection with Borrelia burgdorferi in an immunocompetent patient]. [Article in German]. Dtsch Med Wochenschr 1994; 119(10):338-42.
- Liu NY, Dinerman H, Levin RE, Massarotti E, Molloy PJ, Schoen RT, Taylor E, Steere AC. Randomized trial of doxycycline vs. amoxicillin/probenecid for the treatment of Lyme arthritis: treatment of non responders with iv penicillin or ceftriaxone. Arthritis Rheum 1989, 32: 46.
- Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH 3rd, Liu NY, Schmid CH. Treatment of Lyme arthritis. Arthritis Rheum 1994 Jun; 37(6):878-88.
- Halperin JJ. Abnormalities of the nervous system in Lyme disease: response to antimicrobial therapy. Rev Infect Dis 1989; 11 Suppl 6:S1499-504.
- Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT, Evans J, Weinstein A, Schmid CH, Klempner MS. Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology 2003; 60(12):1916-22.
- Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, Dattwyler R, Chandler B. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 2003; 60(12):1923-30.
- Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 2001; 345(2):85-92.
- Logigian EL, Kaplan RF, Steere AC. Successful treatment of Lyme encephalopathy with intravenous ceftriaxone. J Infect Dis 1999; 180(2):377-83.
- Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to hydroxychloroquine. Int Microbiol 2002; 5(1):25-31.
- Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol 1994; 21(3):454-61.
- Sigal LH. Treatment of Lyme Disease. UpToDate, 2006.
- Ziska MH, Donta ST, Demarest FC. Physician preferences in the diagnosis and treatment of Lyme disease in the United States. Infection 1996; 24(2):182-6.
- Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7-17.
- Manning PG. Fulminant refractory Lyme disease. Iowa Med 1989; 79(6):277-80.
- Limbach FX, Jaulhac B, Puechal X, H Monteil, J Kuntz, Y Piemont, and J Sibilia. Treatment resistant Lyme arthritis caused by Borrelia garinii. Ann Rheum Dis 2001; 60(3):284-6.
- Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, Slavov I, Cheng J, Dobkin J, Nelson DR, Sackeim HA. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008; 70(13):992-1003.
- Duray PH, Steere AC. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci 1988; 539:65-79.
- Oksi J, Nikoskelainen J, Viljanen MK. Comparison of oral cefixime and intravenous ceftriaxone followed by oral amoxicillin in disseminated Lyme borreliosis. Eur J Clin Microbiol Infect Dis 1998; 17(10):715-9.
- Kleemann W, Huismans BD, Heyl S. Prolonged antibiotic therapy in PCR confirmed persistent Lyme disease. Dokument Nr. V166179 01/2011; GRIN VERLAG, Verlag für akademische Texte., ISBN: 9 783640 828210.
- Massengo SA, Bonnet F, Braun C, Vital A, Beylot J, Bastard J. Severe neuroborreliosis: The benefit of prolonged high-dose combination of antimicrobial agents with steroids--an illustrative case. Diagn Microbiol Infect Dis 2005; 51(2):127-30.
- Pfister HW, Preac-Mursic V, Wilske B, Schielke E, Sörgel F, Einhäupl KM. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J Infect Dis 1991; 163(2):311-8.
- Gasser R, Dusleag J. Oral treatment of late borreliosis with roxithromycin plus co-trimoxazole. Lancet 1990; 336(8724):1189-90.
- Hassler D. Langzeitbeobachtungen zum Krankheitsbild der Lyme-Borreliose in einem Endemiegebiet. Habilitationsschrift Universität Erlangen, 1997.
- Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection 1996; 24(3):218-26.
- Strle F, Preac-Mursic V, Cimperman J, Ruzic E, Maraspin V, Jereb M. Azithromycin versus doxycycline for treatment of erythema migrans: clinical and microbiological findings. Infection 1993; 21(2):83-8.
- Mursic VP, Wilske B, Schierz G, Holmburger M, Süss E. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur J Clin Microbiol 1987; 6(4):424-6.
- De Koning J. Histopathologic Aspects of Lyme Borreliosis. Groningen, 145 S., Selbstverlag, 1995.
- Kraiczy P, Skerka C, Kirschfink M, Zipfel PF, Brade V. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int Immunopharmacol 2001; 1(3):393-401.
- Kraiczy P, Skerka C, Kirschfink M, Zipfel PF, Brade V. Immune evasion of Borrelia burgdorferi: insufficient killing of the pathogens by complement and antibody. Int J Med Microbiol 2002; 291 Suppl 33:141-6.
- Kraiczy P, Skerka C, Zipfel PF, Brade V. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: a new protein family involved in complement resistance. Wien Klin Wochenschr 2002; 114(13-14):568-73.
- Kersten A, Poitschek C, Rauch S, Aberer E. Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi. Antimicrob Agents Chemother 1995; 39(5):1127-33.
- Johnson RC. Isolation techniques for spirochetes and their sensitivity to antibiotics in vitro and in vivo. Rev Infect Dis 1989; 11 Suppl 6:S1505-10.
- Hunfeld K-P. Contributions to Seroepidemiology, Diagnosis, and Antimicrobial Susceptibility of Borrelia, Ehrlichia, and Babesia as Indigenous Tick-conducted Pathogens. Shaker Verlag Aachen, Band 2, 2004.
- Kraiczy P. Natürliche Komplementresistenz und humorale Immunabwehr bei Borrelia burgdorferi, dem Erreger der Lyme-Borreliose. Shaker Verlag Aachen, Band 1, 2004.
- Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to metronidazole. APMIS 1999; 107(6):566-76.
- Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection 1996; 24(3):218-26.
- Mattmann LH. Cell Wall Deficient Forms: Stealth pathogens. CRC Press Inc. 3rd ed., 2000.
- Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection 1996; 24(3):218-26.
- Mygland A, Skarpaas T, Ljøstad U. Chronic polyneuropathy and Lyme disease. Eur J Neurol 2006; 13(11):1213-5.
- Robert Koch Institut. Lyme-Borreliose: Zur Situation in den östlichen Bundesländern. Epidemiologisches Bulletin, 38/2007.
- Halperin JJ. Neuroborreliosis. Am J Med 1995; 98(4A):52S-56S.
- Culp RW, Eichenfield AH, Davidson RS, Drummond DS, Christofersen MR, Goldsmith DP. Lyme arthritis in children. An orthopaedic perspective. J Bone Joint Surg Am 1987; 69(1):96-9.
- Horst H. Zeckenborreliose Lyme-Krankheit bei Mensch und Tier. Spitta Verlag, 4. Auflage.
- Satz N. Klinik der Lyme-Borreliose. Verlag Hans Huber, 2. Auflage, 2002.
- Munkelt. Epidemiologische Studie zur Symptomatik, Diagnostik und Therapie der Lyme-Borreliose in Deutschland. Diss. Charité, 2006.
- Mikkilä H, Seppälä I, Leirisalo-Repo M, Immonen I, Karma A. The etiology of uveitis: the role of infections with special reference to Lyme borreliosis. Acta Ophthalmol Scand 1997; 75(6):716-9.
- Leys AM, Schönherr U, Lang GE, Naumann GO, Goubau P, Honore A, Valvekens F. Retinal vasculitis in Lyme borreliosis. Bull Soc Belge Ophtalmol 1995; 259:205-14.
- Winward KE, Smith JL, Culbertson WW, Paris-Hamelin A. Ocular Lyme borreliosis. Am J Ophthalmol 1989; 108(6):651-7.
- Leys AM, Schönherr U, Lang GE, Naumann GO, Goubau P, Honore A, Valvekens F. Retinal vasculitis in Lyme borreliosis. Bull Soc Belge Ophtalmol 1995; 259:205-14.
- Gérard P, Canaple S, Rosa A. [Meningopapillitis disclosing Lyme disease]. [Article in French]. Rev Neurol (Paris) 1996; 152(6-7):476-8.
- Kauffmann DJ, Wormser GP. Ocular Lyme disease: case report and review of the literature. Br J Ophthalmol 1990; 74(6):325-7.
- Sicklinger M, Wienecke R, Neubert U. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J Clin Microbiol 2003; 41(4):1791-3.
- Hohl AF, Frei R, Pünter V, Von Graevenitz A, Knapp C, Washington J, Johnson D, Jones RN. International multicenter investigation of LB20304, a new fluoronaphthyridone. Clin Microbiol Infect 1998; 4(5):280-284.
- Brorson Ø, Brorson SH, Scythes J, MacAllister J, Wier A, Margulis L. Destruction of spirochete Borrelia burgdorferi round-body propagules (RBs) by the antibiotic tigecycline. Proc Natl Acad Sci U S A 2009; 106(44):18656-61.
- Yang X, Nguyen A, Qiu D, Luft BJ. In vitro activity of tigecycline against multiple strains of Borrelia burgdorferi. J Antimicrob Chemother 2009; 63(4):709-12.
- Fallon BA, Levin ES, Schweitzer PJ, Hardesty D. Inflammation and central nervous system Lyme disease. Neurobiol Dis 2010; 37(3):534-41.
- Eskow E, Rao RV, Mordechai E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: evidence for a novel tick-borne disease complex. Arch Neurol 2001; 58(9):1357-63.
- Grab DJ, Nyarko E, Barat NC, Nikolskaia OV, Dumler JS. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin Vaccine Immunol 2007; 14(11):1420-4.
- Mitchell PD, Reed KD, Hofkes JM. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol 1996; 34(3):724-7.
- Oleson CV, Sivalingam JJ, O’Neill BJ, Staas WE Jr. Transverse myelitis secondary to coexistent Lyme disease and babesiosis. J Spinal Cord Med 2003; 26(2):168-71.
- Owen DC. Is Lyme disease always poly microbial?--The jigsaw hypothesis. Med Hypotheses 2006; 67(4):860-4.
- Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from ixodes ticks. Clin Microbiol Rev 2006; 19(4):708-27.
- Thomas V, Anguita J, Barthold SW, Fikrig E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect Immun 2001; 69(5):3359-71.
- Zeidner NS, Dolan MC, Massung R, Piesman J, Fish D. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis suppresses IL-2 and IFN gamma production and promotes an IL-4 response in C3H/HeJ mice. Parasite Immunol 2000; 22(11):581-8.
- Wormser GP, Nadelman RB, Dattwyler RJ, Dennis DT, Shapiro ED, Steere AC, Rush TJ, Rahn DW, Coyle PK, Persing DH, Fish D, Luft BJ. Practice guidelines for the treatment of Lyme disease. The Infectious Diseases Society of America. Clin Infect Dis 2000; 31 Suppl 1:1-14.
- Kristoferitsch W, Stanek G, Kunz C. [Double infection with early summer meningoencephalitis virus and Borrelia burgdorferi]. [Article in German]. Dtsch Med Wochenschr 1986; 111(22):861-4.
- Hunfeld KP, Cinatl KP, Tenter J, Brade A. V.Granulocytic Ehrlichia, Babesia, and spotted fever Rickettsia, Not yet widely known tick-borne pathogens of considerable concern for humans at risk in Europe. Biotest Bulletin 2002; 6:321-344.
- Cadavid D, O’Neill T, Schaefer H, Pachner AR. Localization of Borrelia burgdorferi in the nervous system and other organs in a nonhuman primate model of lyme disease. Lab Invest 2000; 80(7):1043-54.
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089-134.
- Krause PJ, Telford SR 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996; 275(21):1657-60.
- Straubinger RK, Straubinger AF, Summers BA, Jacobson RH. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: An experimental study. J Infect Dis 2000; 181(3):1069-81.
- Stricker RB. Counterpoint: long-term antibiotic therapy improves persistent symptoms associated with lyme disease. Clin Infect Dis 2007; 45(2):149-57.
- YU DT. Reactive arthritis (formerly Reiter syndrome). UpToDate 2009.
- Neubert U, Horst H. Hautmanifestationen, Zeckenborreliose Lyme-Krankheit bei Mensch und Tier. Spitta Verlag, 2003.
- Berger BW. Dermatologic manifestations of Lyme disease. Rev Infect Dis 1989; 11 Suppl 6:S1475-81.
- Aberer E, Klade H. Cutaneous manifestations of Lyme borreliosis. Infection 1991; 19(4):284-6.
- Asbrink E. Cutaneous manifestations of Lyme borreliosis. Clinical definitions and differential diagnoses. Scand J Infect Dis Suppl 1991; 77:44-50.
- Prinz JC, Kutasi Z, Weisenseel P, Pótó L, Battyáni Z, Ruzicka T. “Borrelia-associated early-onset morphea”: a particular type of scleroderma in childhood and adolescence with high titer antinuclear antibodies? Results of a cohort analysis and presentation of three cases. J Am Acad Dermatol 2009; 60(2):248-55.
- Asbrink E, Olsson I. Clinical manifestations of erythema chronicum migrans Afzelius in 161 patients. A comparison with Lyme disease. Acta Derm Venereol 1985; 65(1):43-52.
- Colli C, Leinweber B, Müllegger R, Chott A, Kerl H, Cerroni L. Borrelia burgdorferi-associated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol 2004; 31(3):232-40.
- Trevisan G, Cinco M, Agolzer A. Roseolar lesions in Lyme disease: isolation of the causative agent. Int J Dermatol 1992; 31(7):507-8.
- Trevisan G, Cattonar P, Nobile C, Perkan V, Stinco G. Dermatological Manifestations of Lyme Borreliosis. acta dermatovenerologica, A.P.A., 1996; Vol. 5, 96 No 3-4.
- Oksi J, Marttila H, Soini H, Aho H, Uksila J, Viljanen MK. Early dissemination of Borrelia burgdorferi without generalized symptoms in patients with erythema migrans. APMIS 2001; 109(9):581-8.
- Buechner SA, Lautenschlager S, Itin P, Bircher A, Erb P. Lymphoproliferative responses to Borrelia burgdorferi in patients with erythema migrans, acrodermatitis chronica atrophicans, lymphadenosis benigna cutis, and morphea. Arch Dermatol 1995; 131(6):673-7.
- Aberer E, Klade H. Cutaneous manifestations of Lyme borreliosis. Infection 1991; 19(4):284-6.
- Malane MS, Grant-Kels JM, Feder HM Jr, Luger SW. Diagnosis of Lyme disease based on dermatologic manifestations. Ann Intern Med 1991; 114(6):490-8.
- Ozkan S, Atabey N, Fetil E, Erkizan V, Günes AT. Evidence for Borrelia burgdorferi in morphea and lichen sclerosus. Int J Dermatol 2000; 39(4):278-83.
- Wu YS, Zhang WF, Feng FP, Wang BZ, Zhang YJ. Atypical cutaneous lesions of Lyme disease. Clin Exp Dermatol 1993; 18(5):434-6.
- Goldberg NS, Forseter G, Nadelman RB, Schwartz I, Jorde U, McKenna D, Holmgren D, Bittker S, Montecalvo M, Wormser GP. Vesicular erythema migrans. Arch Dermatol 1992; 128(11):1495-8.
- Hassler D, Zorn J, Zöller L, Neuss M, Weyand C, Goronzy J, Born IA, Preac-Mursic V. [Nodular panniculitis: a manifestation of Lyme borreliosis?]. [Article in German]. Hautarzt 1992; 43(3):134-8.
- Dietrich F, Schmidgen T, Maggi RG, Richter D, Matuschka FR, Vonthein R, Breitschwerdt EB, Kempf VAJ. Prevalence of Bartonella henselae and Borrelia burgdorferi sensu lato DNA in ixodes ricinus ticks in Europe. Appl Environ Microbiol 2010; 76(5):1395-8.
- Spach DH, Kaplan SL. Microbiology, epidemiology, clinical manifestations, and diagnosis of cat scratch disease. UpToDate 2008.
- Schulze J, Sonnenborn U. Yeasts in the gut: from commensals to infectious agents. Dtsch Arztebl Int 2009; 106(51-52):837-42.
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC.Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229-34.
- Straubinger RK. PCR-Based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-Day postinfection period. J Clin Microbiol 2000; 38(6):2191-9.
- Bayer ME, Zhang L, Bayer MH. Borrelia burgdorferi DNA in the urine of treated patients with chronic Lyme disease symptoms. A PCR study of 97 cases. Infection 1996; 24(5):347-53.
- Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, Katz JN, Liang MH. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 1994; 121(8):560-7.
- Liegner KB, Shapiro JR, Ramsay D, Halperin AJ, Hogrefe W, Kong L. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting Borrelia burgdorferi infection. J Am Acad Dermatol 1993; 28(2 Pt 2):312-4.
- Strle F, Maraspin V, Lotric-Furlan S, Ruzić-Sabljić E, Cimperman J. Azithromycin and doxycycline for treatment of Borrelia culture-positive erythema migrans. Infection 1996; 24(1):64-8.
- Hudson BJ, Stewart M, Lennox VA, Fukunaga M, Yabuki M, Macorison H, Kitchener-Smith J. Culture-positive Lyme borreliosis. Med J Aust 1998; 168(10):500-2.
- Battafarano DF, Combs JA, Enzenauer RJ, Fitzpatrick JE. Chronic septic arthritis caused by Borrelia burgdorferi. Clin Orthop Relat Res 1993; (297):238-41.
- Reimers CD, de Koning J, Neubert U, Preac-Mursic V, Koster JG, Müller-Felber W, Pongratz DE, Duray. Borrelia burgdorferi myositis: report of eight patients. J Neurol 1993; 240(5):278-83.
- López-Andreu JA, Ferrís J, Canosa CA, Sala-Lizárraga JV. Treatment of late Lyme disease: a challenge to accept. J Clin Microbiol 1994; 32(5):1415-6.
- Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis 1998; 57(2):118-21.
- Nanagara R, Duray PH, Schumacher HR Jr. Ultrastructural demonstration of spirochetal antigens in synovial fluid and synovial membrane in chronic Lyme disease: possible factors contributing to persistence of organisms. Hum Pathol 1996; 27(10):1025-34.
- Meier P, Blatz R, Gau M, Spencker FB, Wiedemann P. [Pars plana vitrectomy in Borrelia burgdorferi endophthalmitis]. [Article in German]. Klin Monbl Augenheilkd 1998; 213(6):351-4.
- Cimmino MA, Azzolini A, Tobia F, Pesce CM. Spirochetes in the spleen of a patient with chronic Lyme disease. Am J Clin Pathol 1989; 91(1):95-7.
- Hulínská D, Votýpka J, Valesová M. Persistence of Borrelia garinii and Borrelia afzelii in patients with Lyme arthritis. Zentralbl Bakteriol 1999; 289(3):301-18.
- Schoen RT, Aversa JM, Rahn DW, Steere AC. Treatment of refractory chronic Lyme arthritis with arthroscopic synovectomy. Arthritis Rheum 1991; 34(8):1056-60.
- Kirsch M, Ruben FL, Steere AC, Duray PH, Norden CW, Winkelstein A. Fatal adult respiratory distress syndrome in a patient with Lyme disease. JAMA 1988; 259(18):2737-9.
- Aberer E, Kersten A, Klade H, Poitschek C, Jurecka W. Heterogeneity of Borrelia burgdorferi in the skin. Am J Dermatopathol 1996; 18(6):571-9.
- Preac-Mursic V, Pfister HW, Spiegel H, Burk R, Wilske B, Reinhardt S, Böhmer R. First isolation of Borrelia burgdorferi from an iris biopsy. J Clin Neuroophthalmol 1993; 13(3):155-61; discussion 162.
- Hulínská D, Krausová M, Janovská D, Rohácová H, Hancil J, Mailer H. Electron microscopy and the polymerase chain reaction of spirochetes from the blood of patients with Lyme disease. Cent Eur J Public Health 1993; 1(2):81-5.
- Preac Mursic V, Marget W, Busch U, Pleterski Rigler D, Hagl S. Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis. Infection 1996; 24(1):9-16.
- Schmidli J, Hunziker T, Moesli P, Schaad UB. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis 1988; 158(4):905-6.
- Cameron DJ. Clinical trials validate the severity of persistent Lyme disease symptoms. Med Hypotheses 2009; 72(2):153-6.
- Shadick NA, Phillips CB, Sangha O, Logigian EL, Kaplan RF, Wright EA, Fossel AH, Fossel K, Berardi V, Lew RA, Liang MH. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med 1999; 131(12):919-26.
- Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother 2008; 52(5):1728-36.
- Yrjänäinen H, Hytönen J, Söderström KO, Oksi J, Hartiala K, Viljanen MK. Persistent joint swelling and Borrelia-specific antibodies in Borrelia garinii-infected mice after eradication of vegetative spirochetes with antibiotic treatment. Microbes Infect 2006; 8(8):2044-51.
- Feder HM Jr, Whitaker DL. Misdiagnosis of erythema migrans. Am J Med 1995; 99(4):412-9.
- Reid MC, Schoen RT, Evans J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med 1998; 128(5):354-62.
- Steere AC, Taylor E, McHugh GL, Logigian EL. The overdiagnosis of Lyme disease. JAMA 1993; 269(14):1812-6.
- Hassett AL, Radvanski DC, Buyske S, Savage SV, Sigal LH. Psychiatric comorbidity and other psychological factors in patients with “chronic Lyme disease”. Am J Med 2009; 122(9):843-50.
- Vázquez M, Sparrow SS, Shapiro ED. Long-term neuropsychologic and health outcomes of children with facial nerve palsy attributable to Lyme disease. Pediatrics 2003; 112(2):e93-7.
- Klemann W, Huismans BT. Patienten mit Erreger-Direktnachweis bei chronischer Lyme-Borreliose: Klinik, Labordiagnostik, Antibiotika-Therapie und Krankheitsverlauf – Eine retrospektive Studie. umwelt-medizin-gesellschaft, 22, 2/2009
- Logigian EL, Steere AC. Clinical and electrophysiologic findings in chronic neuropathy of Lyme disease. Neurology 1992; 42(2):303-11.
- Sapi E, McDonald A. Biofilms of Borrelia burgdorferi in chronic cutaneous borreliosis. Am J clin Pathol 2008; 129:988-989.
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, Radolf JD. Live imaging reveals a bisphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest 2009; 119:3652-3665.
- Eisendle M, Müller H, Zelger B. Biofilms of Borrelia burgdorferi in chronic cutaneous borreliosis. Am J Clin Pathol 2008; 129:988-989.
- Batsford S, Rust Ch, Neubert U. Analysis of antibody responses to the outer surface protein family in Lyme Borrelosis patients. J Infect Dis 1998; 178:1676-1683.
- Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med 1987; 107:725-731.
- Szer IS, Taylor E, Steere AC. The long-term course of Lyme arthritis in children. N Engl J Med 1991; 325:159-163
- Dattwyler RJ, Volkman DJ, Contay SM, Platkin SP, Luft BJ. Amoxycillin plus probenecid versus doxycycline for treatment of erythema migrans borreliosis. Lancet 1990; 336:1404.
- Luger SW, Parapone P, Wormser GP, Nadelman RB, Grunwaldt E, Gomez G, Wisniewski M, Collins JJ. Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrob Agents Chemother 1995; 39:661.
- Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med 1992; 117:273.
- Luft BJ, Dattwyler RJ, Johnson RC, Luger SW, Bosler EM, Rahn DW, Masters EJ, Grunwaldt E, Gadgil SD. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann Intern Med 1996; 124:785.
- Steere AC, Hutchinson GJ, Rahn DW, Sigal LH, Craft JE, DeSanna ET, Malawista SE.Treatment of the early manifestations of Lyme disease. Ann Intern Med 1983; 99:22.
- Massarotti EM, Luger SW, Rahn DW, Messner RP, Wong JB, Johnson RC, Steere AC. Treatment of early Lyme disease. Am J Med 1992; 92:396.
- Dattwyler RJ, Luft BJ, Kunkel MJ, Finkel MF, Wormser GP, Rush TJ, Grunwaldt E, Agger WA, Franklin M, Oswald D, Cockey L, Maladorno D. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med 1997; 337:289.
- Afzelius A. Verhandlungen der dermatologischen Gesellschaft zu Stockholm Sitzung am 28. Oktober 1909. Arch Dermatol Symph (Berlin) 101 (1919) 404.
- Hauser W. Wahrscheinliche Infektionskrankheiten der Haut. In: Handbuch der Haut- und Geschlechtskrankheiten. Springer-Verlag, Berlin-Heidelberg-New York 1965, 556-629.
- Lipschütz B. Über eine seltene Erythemform (Erythema chronicum migrans). Arch Dermatol Syph 118 (1913), 1482-1486.
- Weber K, Neubert U, Büchner SA. Erythema migrans and early signs and symptoms. In: Weber K, Burgdorfer W: Aspects of Lyme Borreliosis. Springer Verlag, Berlin, 1992, 195-121.
- Strle F, Videncnik J, Zorman P, Cimperman J, Lotric-Furlan S, Maraspin V. Clinical and epidemiological findings for patients with Erythema migrans. Comparison of cohorts from the years 1993 and 2000. Wien Klin Wochenschr 2002, 114:493-497.
- Wu YS, Zhang WF, Feng FP, Wang BZ, Zhang YJ. Atypical cutaneous lesions of Lyme disease. Clin Exp Dermatol 1993; 18(5):434-6.
- Hassler D, Zorn J, Zöller L, Neuss M, Weyand C, Gorozny J, Born IA, Preac-Mursic V. Nodular panniculitis: a manifestation of Lyme borreliosis? Hautarzt 1992; 43(3):134-8.
- Canica MM, Nato F, du Merle L, Mazie JC, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis 1993; 25(4):441-8.
- Ilowite NT. Muscle, reticuloendothelial, and late skin manifestations of Lyme disease. Am J Med 1995; 98(4A):63S-68S.
- Schuttelaar ML, Laeijendecker R, Heinhuis RJ, Van Joost T. Erythema multiforme and persistent erythema as early cutaneous manifestations of Lyme disease. J Am Acad Dermatol 1997; 37(5 Pt 2):873-5.
- Moreno C, Kutzner H, Palmedo G, Goerttler E, Carrasco L, Requena L. Interstitial granulomatous dermatitis with histiocytic pseurorosettes: a new histopathologic pattern in cutaneous borreliosis. Detection of Borrelia burgdorferi DNA sequences by a highly sensitive PCR-ELISA. J Am Acad Dermatol 2003; 47(3):376-84.
- Äsbrink A and Olsson I. Clinical Manifestations of Erythema chronicum migrans Afzelius in 161 Patients. Acta Derm Venerol (Stochk) 1985; 65:42-52.
- Myers SA and Sexton DJ. Dermatologic Manifestations of Arthropod-Borne Diseases. Infectious Disease Clinics of North America 1994, 8(3):689-708.
- Berger BW. Erythema chronicum migrans of Lyme disease. Arch Dermatol 1984; 120:1017-1021.
- Trevisan G, Cinco M. Lyme disease: a general survey. Int J Dermatol 1990; 29:1-8.
- Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, Phuenpathom N, Russanasunthornwong S. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother 1996; 40(3):825-6.
- Hansen K, Hovmark A, Lebech AM, Lebech K, Olsson I, Halkier-Sorensen L, Olsson E, Asbrink E. Roxithromycin in Lyme borreliosis: discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Derm Venerol 1992; 72(4):297-300.
- Sapi E, Kaur N, Anyanwu S, Luecke DF, Datar A, Patel S, Rossi M, Stricker RB. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infection and Drug Resistance 2011; 4 97-113.
- Eisendle K, Zelger B. The expanding spectrum of cutaneous borreliosis. Gital Dermatol Venerol 2009; 114:157-71.
- Centers for Disease Control and Prevention. Atypical Erythema migrans in Patients with PCR-Positive Lyme Disease. Vol. 19, Nr. 5, May 2013.
- BD Huisman, W Klemann. Langzeitbehandlung mit Antiinfektiva bei persistierender Borreliose mit Borrelien-DNA-Nachweis durch PCR. Wissenschaftliche Studie 2008, GRIN-Verlag.
- Nanagara R, Duray PH, Schumacher HR Jr. Ultrastructural demonstration of spirochetal antigens in synovial fluid and synovial membrane in chronic Lyme disease: possible factors contribuiting to persistence of organisms. Hum Pathol 1996; 27(10):1025-34.
- Burgdorfer AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984; 57: 521-525.
- Stricker RB, Burrascano J, Winger E. Longterm decrease in the CD57 lymphocyte subset in a patient with chronic Lyme disease. Ann Agric Environ Med. 2002; 9(1):111-3.
- Brettel H und Vogt H. Ärztliche Begutachtung im Sozialrecht, ecomed - MEDIZIN, 2010.
- References (for appendix)
-
- Satz N. Klinik der Lyme-Borreliose. 3., vollständig überarbeitete und erweiterte Auflage, Verlag Huber.
- Horst H. Zeckenborreliose Lyme-Krankheit bei Mensch und Tier. 4., überarbeitete und erweiterte Auflage, Verlag Spitta.
- Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol 1994; 21(3):454-61.
- Wormser GP, Nadelman RB, Dattwyler RJ, Dennis DT, Shapiro ED, Steere AC, Rush TJ, Rahn DW, Coyle PK, Persing DH, Fish D, Luft BJ. Practice guidelines for the treatment of Lyme disease. The Infectious Diseases Society of America. Clin Infect Dis 2000; 31 Suppl 1:1-14.
- Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med 1990; 323(21):1438-44.
- Fallon BA, Nields JA. Lyme disease: a neuropsychiatric illness. Am J Psychiatry 1994; 151(11):1571-83.
- Halperin JJ. Neuroborreliosis. Am J Med 1995; 98(4A):52S-56S.
- Donta ST. Late and chronic Lyme disease. Med Clin North Am 2002; 86(2):341-9.
- Kalish RA, Kaplan RF, Taylor E, Jones-Woodward L, Workman K, Steere AC. Evaluation of study patients with Lyme disease, 10-20-year follow-up. J Infect Dis 2001; 183(3):453-60.
- Horst H. Einheimische Zeckenborreliose bei Mensch und Tier (2. Auflage). Perimed-Verlag.
- Meier C, Reulen HJ, Huber P, Mumenthaler M. Meningoradiculoneuritis mimicking vertebral disc herniation. A “neurosurgical” complication of Lyme-borreliosis. Acta Neurochir (Wien) 1989; 98(1-2):42-6.
- Ackermann R, Gollmer E, Rehse-Küpper B. [Progressive Borrelia encephalomyelitis. Chronic manifestation of erythema chronicum migrans disease of the nervous system]. [Article in German]. Dtsch Med Wochenschr 1985; 110(26):1039-42.
- Barnett W, Sigmund D, Roelcke U, Mundt C. [Endogenous paranoid-hallucinatory syndrome caused by Borrelia encephalitis]. [Article in German]. Nervenarzt 1991; 62(7):445-7.
- Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med 1990; 323(21):1438-44.
- Herzer P, Wilske B. Lyme arthritis in Germany. Zentralbl Bakteriol Mikrobiol Hyg A 1986; 263(1-2):268-74.
- Stanek G, Klein J, Bittner R, Glogar D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med 1990; 322(4):249-52.
- van der Linde MR, Crijns HJ, de Koning J, Hoogkamp-Korstanje JA, De Graaf JJ, Piers DA, van der Galiën A, Lie KI.Range of atrioventricular conduction disturbances in Lyme borreliosis: a report of four cases and review of other published reports. Br Heart J 1990; 63(3):162-8.
- Asbrink E, Hovmark A, Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol 1984; 64(6):506-12.
- Langer K, Diem E. [Acrodermatitis chronic atrophicans and sclerodermiform skin changes in Borrelia infection]. [Article in German]. Hautarzt 1988; 39(10):647-51.
- Garbe C, Stein H, Gollnick H, Taud W, Orfanos CE. [Cutaneous B cell lymphoma in chronic Borrelia burgdorferi infection. Report of 2 cases and a review of the literature]. [Article in German]. Hautarzt 1988; 39(11):717-26.
- Hassler D, Zorn J, Zöller L, Neuss M, Weyand C, Goronzy J, Born IA, Preac-Mursic V. [Nodular panniculitis: a manifestation of Lyme borreliosis?]. [Article in German]. Hautarzt 1992; 43(3):134-8.
- McDonald AB. Gestational Lyme borreliosis. Implications for the fetus. Rheum Dis Clin North Am 1989; 15(4):657-77.
- Winterkorn JM. Lyme disease: neurologic and ophthalmic manifestations. Surv Ophthalmol 1990; 35(3):191-204.
- Mokry M, Flaschka G, Kleinert G, Kleinert R, Fazekas F, Kopp W. Chronic Lyme disease with an expansive granulomatous lesion in the cerebellopontine angle. Neurosurgery 1990; 27(3):446-51.
- Rank EL, Dias SM, Hasson J, Duray PH, Johnson RC, Magnarelli LA, Fister RD. Human necrotizing splenitis caused by Borrelia burgdorferi. Am J Clin Pathol 1989; 91(4):493-8.
- Schoenen J, Sianard-Gainko J, Carpentier M, Reznik M. Myositis during Borrelia burgdorferi infection (Lyme disease). J Neurol Neurosurg Psychiatry 1989; 52(8):1002-5.
- Duray PH. Clinical pathologic correlations of Lyme disease. Rev Infect Dis 1989; 11 Suppl 6:S1487-93.
- Halperin JJ, Little BW, Coyle PK, Dattwyler RJ. Lyme disease: cause of a treatable peripheral neuropathy. Neurology 1987; 37(11):1700-6.
- Halperin JJ, Luft BJ, Anand AK Roque CT, Alvarez O, Volkman DJ, Dattwyler RJ. Lyme neuroborreliosis: central nervous system manifestations. Neurology 1989; 39(6):753-9.
- Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med 1990; 323(21):1438-44.
- Bransfield RC, Wulfman JS, Harvey WT, Usman AI. The association between tick-borne infections, Lyme borreliosis and autism spectrum disorders. Med Hypotheses 2008; 70(5):967-74.
- Berger BW. Dermatologic manifestations of Lyme disease. Rev Infect Dis 1989; 11 Suppl 6:S1475-81.
- Asbrink E, Olsson I. Clinical manifestations of erythema chronicum migrans Afzelius in 161 patients. A comparison with Lyme disease. Acta Derm Venereol 1985; 65(1):43-52.
- Trevisan G, Cattonar P, Nobile C, Perkan V, Stinco G. Dermatological Manifestations of Lyme Borreliosis. Acta Dermatovenerologica 1996; A.P.A. Vol. 5, 96 No 3-4.
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med 1995; 98(4A):60S-62S.
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin 1993; 33(1):9-22.
- Mikkilä H, Seppälä I, Leirisalo-Repo M, Immonen I, Karma A. The etiology of uveitis: the role of infections with special reference to Lyme borreliosis. Acta Ophthalmol Scand 1997; 75(6):716-9.
- Finizia C, Jönsson R, Hanner P. Serum and cerebrospinal fluid pathology in patients with sudden sensorineural hearing loss. Acta Otolaryngol 2001; 121(7):823-30.